Rab38 and Rab32 control post-Golgi trafficking of melanogenic enzymes - PubMed (original) (raw)
Rab38 and Rab32 control post-Golgi trafficking of melanogenic enzymes
Christina Wasmeier et al. J Cell Biol. 2006.
Abstract
A mutation in the small GTPase Rab38 gives rise to the mouse coat color phenotype "chocolate" (cht), implicating Rab38 in the regulation of melanogenesis. However, its role remains poorly characterized. We report that cht Rab38(G19V) is inactive and that the nearly normal pigmentation in cht melanocytes results from functional compensation by the closely related Rab32. In cht cells treated with Rab32-specific small interfering RNA, a dramatic loss of pigmentation is observed. In addition to mature melanosomes, Rab38 and Rab32 localize to perinuclear vesicles carrying tyrosinase and tyrosinase-related protein 1, consistent with a role in the intracellular sorting of these proteins. In Rab38/Rab32-deficient cells, tyrosinase appears to be mistargeted and degraded after exit from the trans-Golgi network (TGN). This suggests that Rab38 and Rab32 regulate a critical step in the trafficking of melanogenic enzymes, in particular, tyrosinase, from the TGN to melanosomes. This work identifies a key role for the Rab38/Rab32 subfamily of Rab proteins in the biogenesis of melanosomes and potentially other lysosome-related organelles.
Figures
Figure 1.
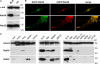
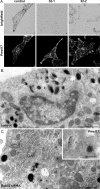
Pigmentation in melanocytes carrying the cht mutation and biochemical characterization of cht mutant Rab38G19V. (A) Brightfield images of control +/cht and homozygous cht/cht primary skin melanocytes at 1–5 wk in culture. (B) Primary melanocytes cultured for 5 wk were processed for conventional EM. Sections show cytoplasmic organelles near the cell periphery. Bar, 500 nm. (C) Brightfield images of immortal melanocyte cell lines derived from BL/6 (melan-Ink4a) or cht (melan-cht) mice carrying an Ink4a deletion. (D) BL/6 or cht melanocyte homogenates were separated into soluble (S) and pelletable (P) fractions by centrifugation at 100,000 g (100K) or into aqueous (A) and detergent (D) fractions by extraction with Triton X-114 (TX-114). The distribution of Rab38, or Rab27a as a control, was analyzed by immunoblotting. (E) BL/6 cells were transiently transfected with EGFP-Rab38 or -Rab38G19V, as indicated. EGFP fluorescence and the corresponding phase-contrast images are shown. (right) Boxed regions at higher magnification, with pigment represented by an inverted phase-contrast image.
Figure 2.
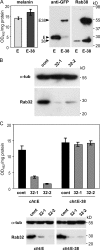
Expression pattern and subcellular localization of Rab38 and Rab32. (A) Proteins from BL/6 or cht melanocytes were separated by SDS-PAGE and immunoblotted for Rab38, Rab32, and α-tubulin (α-tub) as loading control. (B) Melanocytes were transiently cotransfected with EGFP-Rab32 and mRFP-Rab38. Green and red fluorescent signals are shown, and colocalization is represented in yellow in the merged images. (bottom) A cell process at higher magnification. (C) Proteins from mouse tissues (50 μg/lane) or from a range of cultured mouse or rat cell lines available in our laboratory (25 μg/lane) were separated by SDS-PAGE and immunoblotted for Rab38, Rab32, and tubulin as loading control. (right and left) α-Tubulin; (middle) γ-tubulin. The asterisk indicates a nonspecific band in the Rab32 panels.
Figure 3.
Rab38 and Rab32 are required for melanocyte pigmentation and act in a functionally redundant way. (A) EGFP (E) or EGFP-Rab38 (E-38) were stably overexpressed in cht cells using lentiviral vectors. Cellular proteins were immunoblotted for EGFP or Rab38, as indicated. Melanin content was assayed by measuring optical density at 492 nm (OD492). Assays were performed in triplicate; means and standard deviations are depicted. (B) Cht cells were transfected with either control siRNA oligos or Rab32-specific siRNA oligos (32-1 or 32-2). Cellular proteins were immunoblotted for Rab32 and α-tubulin. (C) Cht cells expressing EGFP (cht:E) or EGFP-Rab38 (cht:E-38) were subjected to two rounds of transfection with control or Rab32-specific siRNA oligos. Melanin content was measured on day 10 after the initial transfection. Assays were performed in triplicate, as above. Cells were also analyzed for expression of Rab32 by immunoblotting (bottom).
Figure 4.
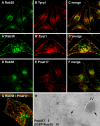
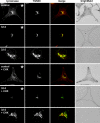
Rab38 colocalizes with melanosomal proteins in peripheral melanosomes and in perinuclear vesicles. BL/6 melanocytes were transiently transfected with EGFP-Rab38 (EGFP-38; A and F). Cells were fixed, permeabilized, and labeled with antibodies to tyrosinase (B) or Tyrp1 (G), as indicated. (C and H) Merged fluorescent images; (D and I) corresponding phase-contrast images; (E and J) Fluorescent signals and phase contrast (blue) are merged. Panels show regions of cells that include perinuclear and peripheral areas, and insets represent the whole cell. (K–O) Labeled structures at the cell periphery are depicted at higher magnification; EGFP-Rab38 (K), pigment (L), Tyrp1 (N), and merged images (M and O) are shown. (P–W) Melanocytes were cotransfected with EGFP-Rab27a (P and T) and mRFP-Rab38 (Q and U). (R and V) Merged images; (S and W) phase contrast.
Figure 5.
Rab38-positive structures are distinct from stage II melanosomes. MNT-1 cells were fixed, permeabilized, and double labeled with antibodies to Rab38 (A and D) and either Tyrp1 (B and C) or Pmel17 (HMB45; E–G). (C, F, and G) Merged images; (A′–C′ and G) higher magnifications. (H) MNT-1 cells were transduced with lentivirus to stably express EGFP-Rab38 and were processed for immuno-EM. Ultrathin cryosections were double labeled with antibodies to EGFP (10-nm gold) and Pmel17 (5-nm gold). Stage II and IV melanosomes (arrows) are indicated.
Figure 6.
Distribution of Rab38, Tyrp1, and tyrosinase on small cytoplasmic vesicles and tubules. (A) Ultrathin sections of MNT-1 cells stably expressing EGFP-Rab38 were labeled with antibodies to EGFP or EGFP (10-nm gold) and Tyrp1 (5-nm gold), as indicated. EGFP-Rab38 is present on stage IV melanosomes and on vesicles and tubules (arrows) near melanosomes and the Golgi (GA). (B) BL/6 melanocytes stably expressing EGFP-Rab38 were labeled with antibodies to EGFP or EGFP (10-nm gold) and tyrosinase (5-nm gold), as indicated. Bars, 200 nm.
Figure 7.
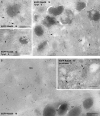
Loss of mature melanosomes but not stage II organelles in Rab38/Rab32-deficient cells. (A) Cht melanocytes subjected to two rounds of transfection with either control or Rab32-specific siRNA oligos were fixed, permeabilized, and labeled for Pmel17 with antibody HMB45. Note the reduction in pigmented structures in the corresponding brightfield images of Rab32 siRNA–treated cells. (B) Cht cells transfected with control siRNA were processed for conventional EM. Melanosomes at different stages of maturation are indicated. (C) Cht cells transfected with Rab32-specific siRNA were analyzed as in B. The inset shows immunogold labeling for Pmel17 on stage II melanosomes in cht cells treated with Rab32-specific siRNA and processed for immuno-EM. N, nucleus; GA, Golgi. Bars: (B and C) 500 nm; (inset) 200 nm.
Figure 8.
Tyrosinase and Tyrp1 trafficking to the melanosome is blocked in Rab38/Rab32-deficient cells. Cht melanocytes transfected with either control or Rab32-specific siRNA oligos as in Fig. 7 were fixed, permeabilized, and immunolabeled for Tyrp1 and tyrosinase, as indicated. The corresponding brightfield images are shown in the top panels.
Figure 9.
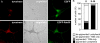
Localization of tyrosinase to the TGN in control and Rab38/Rab32-deficient cells. Control or Rab32 (32-2) siRNA–treated cht cells were either fixed under steady-state conditions (top) or were incubated with cycloheximide for 3 h before fixation (bottom; + CHX). Cells were permeabilized and double labeled for tyrosinase (red) and TGN38 (green), as indicated. Colocalization is shown as yellow in the merged images. The outline of depigmented Rab32 knockdown cells is depicted in the brightfield images. For comparison of tyrosinase levels between panels marked with asterisks, images of tyrosinase labeling were acquired using the same microscope settings; for analysis of colocalization with TGN38 in images showing higher magnifications, signal intensities were adjusted independently.
Figure 10.
Expression of exogenous Rab38 rescues tyrosinase trafficking in Rab38/Rab32-deficient cells. (A) Cht melanocytes were subjected to two rounds of transfection with Rab32-specific siRNA oligos. On day 6 after the initial transfection, cells were transduced with lentivirus expressing either EGFP or EGFP-Rab38, as indicated. 4 d later, cells were fixed, permeabilized, and labeled for tyrosinase (red). The corresponding brightfield images and EGFP fluorescence (green) are also shown. (B) EGFP (E) or EGFP-Rab38 (E-38) positive cells were scored as either pigmented or depigmented, and tyrosinase was categorized as either confined to the perinuclear region (TGN only) or present in vesicular structures at the cell periphery (peripheral). Numbers of cells in each category are plotted as a percentage of the total number of cells counted (n = 197 cells for EGFP; n = 126 cells for EGFP-Rab38).
Comment in
- Darkness descends with two Rabs.
Marks MS. Marks MS. J Cell Biol. 2006 Oct 23;175(2):199-200. doi: 10.1083/jcb.200608058. Epub 2006 Oct 16. J Cell Biol. 2006. PMID: 17043141 Free PMC article.
Similar articles
- RUTBC1 Functions as a GTPase-activating Protein for Rab32/38 and Regulates Melanogenic Enzyme Trafficking in Melanocytes.
Marubashi S, Shimada H, Fukuda M, Ohbayashi N. Marubashi S, et al. J Biol Chem. 2016 Jan 15;291(3):1427-40. doi: 10.1074/jbc.M115.684043. Epub 2015 Nov 30. J Biol Chem. 2016. PMID: 26620560 Free PMC article. - BLOC-2, AP-3, and AP-1 proteins function in concert with Rab38 and Rab32 proteins to mediate protein trafficking to lysosome-related organelles.
Bultema JJ, Ambrosio AL, Burek CL, Di Pietro SM. Bultema JJ, et al. J Biol Chem. 2012 Jun 1;287(23):19550-63. doi: 10.1074/jbc.M112.351908. Epub 2012 Apr 16. J Biol Chem. 2012. PMID: 22511774 Free PMC article. - Mutation of melanosome protein RAB38 in chocolate mice.
Loftus SK, Larson DM, Baxter LL, Antonellis A, Chen Y, Wu X, Jiang Y, Bittner M, Hammer JA 3rd, Pavan WJ. Loftus SK, et al. Proc Natl Acad Sci U S A. 2002 Apr 2;99(7):4471-6. doi: 10.1073/pnas.072087599. Epub 2002 Mar 26. Proc Natl Acad Sci U S A. 2002. PMID: 11917121 Free PMC article. - Cell type-specific Rab32 and Rab38 cooperate with the ubiquitous lysosome biogenesis machinery to synthesize specialized lysosome-related organelles.
Bultema JJ, Di Pietro SM. Bultema JJ, et al. Small GTPases. 2013 Jan-Mar;4(1):16-21. doi: 10.4161/sgtp.22349. Epub 2012 Dec 17. Small GTPases. 2013. PMID: 23247405 Free PMC article. Review. - Rab GTPases: Key players in melanosome biogenesis, transport, and transfer.
Fukuda M. Fukuda M. Pigment Cell Melanoma Res. 2021 Mar;34(2):222-235. doi: 10.1111/pcmr.12931. Epub 2020 Oct 14. Pigment Cell Melanoma Res. 2021. PMID: 32997883 Review.
Cited by
- BLOC-2 targets recycling endosomal tubules to melanosomes for cargo delivery.
Dennis MK, Mantegazza AR, Snir OL, Tenza D, Acosta-Ruiz A, Delevoye C, Zorger R, Sitaram A, de Jesus-Rojas W, Ravichandran K, Rux J, Sviderskaya EV, Bennett DC, Raposo G, Marks MS, Setty SR. Dennis MK, et al. J Cell Biol. 2015 May 25;209(4):563-77. doi: 10.1083/jcb.201410026. J Cell Biol. 2015. PMID: 26008744 Free PMC article. - Chitinase 3-like-1 and its receptors in Hermansky-Pudlak syndrome-associated lung disease.
Zhou Y, He CH, Herzog EL, Peng X, Lee CM, Nguyen TH, Gulati M, Gochuico BR, Gahl WA, Slade ML, Lee CG, Elias JA. Zhou Y, et al. J Clin Invest. 2015 Aug 3;125(8):3178-92. doi: 10.1172/JCI79792. Epub 2015 Jun 29. J Clin Invest. 2015. PMID: 26121745 Free PMC article. Clinical Trial. - Modular metabolite assembly in Caenorhabditis elegans depends on carboxylesterases and formation of lysosome-related organelles.
Le HH, Wrobel CJ, Cohen SM, Yu J, Park H, Helf MJ, Curtis BJ, Kruempel JC, Rodrigues PR, Hu PJ, Sternberg PW, Schroeder FC. Le HH, et al. Elife. 2020 Oct 16;9:e61886. doi: 10.7554/eLife.61886. Elife. 2020. PMID: 33063667 Free PMC article. - Assembly of the biogenesis of lysosome-related organelles complex-3 (BLOC-3) and its interaction with Rab9.
Kloer DP, Rojas R, Ivan V, Moriyama K, van Vlijmen T, Murthy N, Ghirlando R, van der Sluijs P, Hurley JH, Bonifacino JS. Kloer DP, et al. J Biol Chem. 2010 Mar 5;285(10):7794-804. doi: 10.1074/jbc.M109.069088. Epub 2010 Jan 4. J Biol Chem. 2010. PMID: 20048159 Free PMC article. - Genome-wide mapping of signatures of selection using a high-density array identified candidate genes for growth traits and local adaptation in chickens.
Mastrangelo S, Ben-Jemaa S, Perini F, Cendron F, Biscarini F, Lasagna E, Penasa M, Cassandro M. Mastrangelo S, et al. Genet Sel Evol. 2023 Mar 23;55(1):20. doi: 10.1186/s12711-023-00790-6. Genet Sel Evol. 2023. PMID: 36959552 Free PMC article.
References
- Bao, X., A.E. Faris, E.K. Jang, and R.J. Haslam. 2002. Molecular cloning, bacterial expression and properties of Rab31 and Rab32. Eur. J. Biochem. 269:259–271. - PubMed
- Bennett, D.C., and M.L. Lamoreux. 2003. The color loci of mice—a genetic century. Pigment Cell Res. 16:333–344. - PubMed
- Cohen-Solal, K.A., R. Sood, Y. Marin, S.M. Crespo-Carbone, D. Sinsimer, J.J. Martino, C. Robbins, I. Makalowska, J. Trent, and S. Chen. 2003. Identification and characterization of mouse Rab32 by mRNA and protein expression analysis. Biochim. Biophys. Acta. 1651:68–75. - PubMed
- Demaison, C., K. Parsley, G. Brouns, M. Scherr, K. Battmer, C. Kinnon, M. Grez, and A.J. Thrasher. 2002. High-level transduction and gene expression in hematopoietic repopulating cells using a human immunodeficiency [correction of immunodeficiency] virus type 1-based lentiviral vector containing an internal spleen focus forming virus promoter. Hum. Gene Ther. 13:803–813. - PubMed
- Fujikawa, K., A.K. Satoh, S. Kawamura, and K. Ozaki. 2002. Molecular and functional characterization of a unique Rab protein, RABRP1, containing the WDIAGQE sequence in a GTPase motif. Zoolog. Sci. 19:981–993. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous