E2F4 regulates a stable G2 arrest response to genotoxic stress in prostate carcinoma - PubMed (original) (raw)
E2F4 regulates a stable G2 arrest response to genotoxic stress in prostate carcinoma
M E Crosby et al. Oncogene. 2007.
Abstract
The retinoblastoma (pRB) family proteins regulate the E2F transcription factors; their complexes regulate critical transitions through the cell cycle. The function of these pRB family/E2F complexes, which includes p130/E2F4, in response to genotoxic agents, is not well understood. We investigated the role of E2F4 in the genotoxic stress response. Following radiation treatment, E2F4 colocalized with p130 in the nucleus during a radiation-induced stable G(2)-phase arrest. Arrested cells had significantly decreased expression of Cyclins A2 and B1 and decreased phosphorylation of mitotic protein monoclonal-2 (MPM-2) mitotic proteins. Small interference RNA (siRNA)-mediated knockdown of E2F4 sensitized cells to subsequent irradiation, resulting in enhanced cellular DNA damage and cell death, as determined by caspase activation and decreased clonogenic cell survival. Downstream E2F4 targets potentially involved in the progression from G(2) into M phase were identified by oligonucleotide microarray expression profiling. Chromatin immunoprecipitation localized E2F4 at promoter regions of the Bub3 and Pttg1 mitotic genes following irradiation, which were among the downregulated genes identified by the microarray. These data suggest that in response to radiation, E2F4 becomes active in the nucleus, enforces a stable G(2) arrest by target gene repression, and thus provides increased cell survival ability by minimizing propagation of cells that have irreparable DNA damage.
Figures
Figure 1
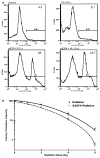
Radiation induces a robust cell-cycle arrest in G2 and M independently of p53 function in prostate carcinoma C4-2 cells. Flow cytometry analyses, by staining for DNA content at different time points following irradiation, were performed as described in ‘Materials and methods.’ Percentages of C4-2 (a) and DN-p53-C4- 2 (b) cells in the different phases of the cell cycle analysed by flow cytometry are presented with respect to time. Data are representative of three separate and independent experiments.
Figure 2
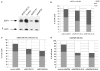
Radiation-induced cell cycle arrest is specific to G2 phase. (a) Multiparametric staining was performed using the mitotic marker, MPM-2 (FSE, _y_-axis), Cyclin A2 (PE, _y_-axis), Cyclin B1 (Alexa 647, _y_-axis), and Hoechst 33342 (_x_-axis) for DNA content. The proportion of mitotic cells, highlighted with an arrow, was examined at the indicated times following irradiation. The light scatter profiles shown in the upper right-hand corners of the Cyclin A2 plot indicate that this population of A−/B− state is not apoptotic. (b) Proportion of cells in G1, S, and G2/M phase. (c) Bivariate analyses examined staining for Cyclin A2 and B1 in cells retaining 4C DNA content. For each staining combination and analysis, cells were collected before (0 h) and at 6, 12, and 24 h after irradiation. (d) Immunoblotting for Cyclin A, B1, and PTTG1 was performed using the respective primary antibodies. _β_-Actin was used as a loading control.
Figure 3
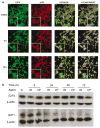
E2F4 has sustained levels after irradiation and colocalizes with p130 at the time of G2 arrest. (a) Immunofluoresscence confocal microscopy (×40 magnification) with antibodies specific for E2F4 and p130 indicated colocalization following irradiation. Merging the fluorescence signals provided by the two antibodies indicated their nuclear colocalization following irradiation. (b) irradiation and VP-16 treatments lead to sustained levels of E2F4 protein expression. C4-2 cells were left untreated (control), treated with 10 _μ_M VP-16 or irradiated, and harvested at the time points indicated. Proteins in cell lysates were resolved by 10% SDS– PAGE, before immunoblotting with anti-E2F4 and -E2F-1 antibodies, with _β_-actin used as a loading control.
Figure 4
E2F4 downregulation causes abrogation of radiation-induced G2 cell cycle arrest and an apparent increase in S. (a) Cells were treated with vehicle control, siGAPDH, an ineffective or a selective siRNA against E2F4 at 24 h before collection and immunobloting, indicating that the specific siRNA against E2F4 (#1) was highly effective. (b) A non-specific siRNA to E2F4 [siE2f4 (I)] or specific to GAPDH (d) did not cause a marked decrease in the G2 arrest compared to data above (Figure 1a). (c) No changes in 4C and an apparent increase in the S-phase population in cells treated with siE2F4 before irradiation.
Figure 5
Lack of BrdU incorporation reveals apoptotic cells with sub-G1 and sub-G2 DNA content. (a) A sub-G1 population of cells is generated at 12 h following radiation treatment in cells that have been transfected with siE2F4 24 h before irradiation. (b) Dual staining for incorporation of BrdU and DNA content following transfection and irradiation was performed to eliminate the possibility that the cells were re-entering S phase. Insets indicate the gating used for identifying BrdU+ cells (% in upper left-hand corner). (c) Cells were treated with vehicle alone or siE2F4, incubated for 24 h, irradiated, and labeled for 45 min before collection at either 8 or 16 h following irradiation. Although control cells show active incorporation of BrdU, those transfected and collected at 16 h following irradiation had a BrdU-negative S-phase population.
Figure 6
E2F4 depletion leads to caspase activation and loss of clonogenic survival. (a) The CaspaTag™ assay was used to determine proteolytic caspase activation that results in free cysteine residues that covalently bind to the fluorescent substrate. (b) After treatment with siE2F4, cells were re-plated logarithmically (100–2500 cells/ml). After a 10-day incubation, colonies were fixed, stained and examined with respect to the untreated control. The significance was tested between irradiation and siE2F4+ irradiation by _F_-test using Prism software. Data represent the mean±s.d. of triplicate experiments.
Figure 7
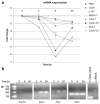
Irradiation induces downregulation of E2F4 target genes. (a) Quantitative RT-PCR was performed to validate the levels of mRNA of genes identified by the microarray (Table 1) using the primers described in Table 2 (Materials and methods) at various time points following irradiation. The data are expressed with respect to the endogenous GAPDH control, values reported being relative to the 0 h time point. (b) ChIP analyses confirmed dynamic target binding to Bub3 and Pttg1 genes and constitutive binding to CDC6. β-Actin primers were used to confirm that there was no non-specific E2F4 binding and to serve as a positive control for the ‘Total input.’ In the ‘Mock’ control, ChIP was carried out without chromatin as a control for non-specific binding.
Similar articles
- Genotoxic stress induces expression of E2F4, leading to its association with p130 in prostate carcinoma cells.
DuPree EL, Mazumder S, Almasan A. DuPree EL, et al. Cancer Res. 2004 Jul 1;64(13):4390-3. doi: 10.1158/0008-5472.CAN-03-3695. Cancer Res. 2004. PMID: 15231644 - E2F4 function in G2: maintaining G2-arrest to prevent mitotic entry with damaged DNA.
Plesca D, Crosby ME, Gupta D, Almasan A. Plesca D, et al. Cell Cycle. 2007 May 15;6(10):1147-52. doi: 10.4161/cc.6.10.4259. Epub 2007 May 11. Cell Cycle. 2007. PMID: 17507799 Free PMC article. Review. - E2F mediates sustained G2 arrest and down-regulation of Stathmin and AIM-1 expression in response to genotoxic stress.
Polager S, Ginsberg D. Polager S, et al. J Biol Chem. 2003 Jan 17;278(3):1443-9. doi: 10.1074/jbc.M210327200. Epub 2002 Nov 21. J Biol Chem. 2003. PMID: 12446714 - E2F-HDAC complexes negatively regulate the tumor suppressor gene ARHI in breast cancer.
Lu Z, Luo RZ, Peng H, Huang M, Nishmoto A, Hunt KK, Helin K, Liao WS, Yu Y. Lu Z, et al. Oncogene. 2006 Jan 12;25(2):230-9. doi: 10.1038/sj.onc.1209025. Oncogene. 2006. PMID: 16158053 - TGFbeta-mediated formation of pRb-E2F complexes in human myeloid leukemia cells.
Hu XT. Hu XT. Biochem Biophys Res Commun. 2008 May 2;369(2):277-80. doi: 10.1016/j.bbrc.2008.02.051. Epub 2008 Feb 21. Biochem Biophys Res Commun. 2008. PMID: 18294958 Review.
Cited by
- Tumor microenvironment deconvolution identifies cell-type-independent aberrant DNA methylation and gene expression in prostate cancer.
Reynolds SR, Zhang Z, Salas LA, Christensen BC. Reynolds SR, et al. Clin Epigenetics. 2024 Jan 3;16(1):5. doi: 10.1186/s13148-023-01609-3. Clin Epigenetics. 2024. PMID: 38173042 Free PMC article. - Identification of lncRNAs involved in response to ionizing radiation in fibroblasts of long-term survivors of childhood cancer and cancer-free controls.
Grandt CL, Brackmann LK, Poplawski A, Schwarz H, Marini F, Hankeln T, Galetzka D, Zahnreich S, Mirsch J, Spix C, Blettner M, Schmidberger H, Marron M. Grandt CL, et al. Front Oncol. 2023 Apr 27;13:1158176. doi: 10.3389/fonc.2023.1158176. eCollection 2023. Front Oncol. 2023. PMID: 37182169 Free PMC article. - Distinct mechanisms mediating therapy-induced cellular senescence in prostate cancer.
Kallenbach J, Atri Roozbahani G, Heidari Horestani M, Baniahmad A. Kallenbach J, et al. Cell Biosci. 2022 Dec 15;12(1):200. doi: 10.1186/s13578-022-00941-0. Cell Biosci. 2022. PMID: 36522745 Free PMC article. Review. - A Novel Strategy to Identify Prognosis-Relevant Gene Sets in Cancers.
Pu J, Yu H, Guo Y. Pu J, et al. Genes (Basel). 2022 May 12;13(5):862. doi: 10.3390/genes13050862. Genes (Basel). 2022. PMID: 35627247 Free PMC article. - Differential Expression of E2F Transcription Factors and Their Functional and Prognostic Roles in Human Prostate Cancer.
Han Z, Mo R, Cai S, Feng Y, Tang Z, Ye J, Liu R, Cai Z, Zhu X, Deng Y, Zou Z, Wu Y, Cai Z, Liang Y, Zhong W. Han Z, et al. Front Cell Dev Biol. 2022 Apr 21;10:831329. doi: 10.3389/fcell.2022.831329. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35531101 Free PMC article.
References
- Almasan A, Linke S, Paulson T, Huang L-c, Wahl GM. Genetic instability as a consequence of inappropriate entry and progression through S-phase. Cancer Metast Rev. 1995a;14:59–73. - PubMed
- Bindra RS, Gibson SL, Meng A, Westermark U, Jasin M, Pierce AJ, et al. Hypoxia-induced down-regulation of BRCA1 expression by E2Fs. Cancer Res. 2005;65:11597–11604. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA081504-09/CA/NCI NIH HHS/United States
- R01 CA081504-07/CA/NCI NIH HHS/United States
- CA82858/CA/NCI NIH HHS/United States
- R01 CA082858/CA/NCI NIH HHS/United States
- CA81504/CA/NCI NIH HHS/United States
- R01 CA081504/CA/NCI NIH HHS/United States
- R01 CA081504-08/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous