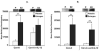IFN-gamma-inducing transcription factor, T-bet is upregulated by estrogen in murine splenocytes: role of IL-27 but not IL-12 - PubMed (original) (raw)
IFN-gamma-inducing transcription factor, T-bet is upregulated by estrogen in murine splenocytes: role of IL-27 but not IL-12
Ebru Karpuzoglu et al. Mol Immunol. 2007 Mar.
Abstract
Estrogen is believed to be involved in regulation of the differentiation, survival, or function of diverse immune cells as well as in many autoimmune and inflammatory diseases. However, the mechanisms behind the immunomodulatory effects of estrogen are poorly understood. Previously, we have shown that natural estrogen can upregulate IFN-gamma and IFN-gamma-mediated-inflammatory events (iNOS, nitric oxide, COX-2). Since IFN-gamma is regulated by T-bet, in this study, we investigated whether estrogen induces T-bet expression in primary murine splenocytes. We found that in vivo estrogen treatment primes splenocytes for early upregulation of T-bet upon activation by T cell stimulants, Concanavalin-A (Con-A) or anti-CD3 antibodies. The expression of T-bet protein was not altered by IL-12 while IFN-gamma had partial effects on T-bet in splenocytes from estrogen-treated mice. Notably, T-bet expression increased in Con-A-activated splenocytes from estrogen-treated mice in the presence of IL-27. Together, our studies show that in vivo estrogen exposure primes lymphocytes towards Th1 type development by promoting/upregulating T-bet expression, which is upregulated in part by IFN-gamma and IL-27. Given that T-bet is a potent inducer of IFN-gamma, these studies may lead to new lines of investigation in relation to many female-predominant autoimmune diseases and inflammatory disorders.
Figures
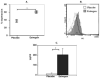
Figure 1. Estrogen upregulates early production of IFN-γ protein
(A) Intracellular IFN-γ protein was detected in freshly isolated lymphocytes from estrogen and placebo-treated mice. The cells were stained with anti-IFN-γ antibodies and evaluated by flow cytometry. The relative expression of IFN-γ-stained cells was presented as means ± SEM (standard errors of mean) (placebo: n=5 mice, estrogen: n=5 mice; *p<0.05). (B) Representative histograms from placebo and estrogen-treated mice are overlayed on top of each other. The faint unidentified histogram represents background control. (C) The protein levels of IFN-γ were determined in the supernatants from Concanavalin-A (Con-A; 10 μg/ml) -stimulated or unstimulated (media only) splenic lymphocytes from estrogen or placebo-treated mice. Extracellular protein levels of IFN-γ were upregulated as early as 3 hrs (placebo: n=4 mice, estrogen: n=4 mice; *p<0.05). Data are presented as means with standard errors of the mean.There was no detectable IFN-γ in supernatants from unstimulated cultures (media only).
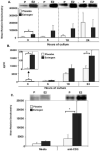
Figure 2. Estrogen upregulates T-bet protein expression in activated splenic lymphocytes in a time-dependent fashion
(A) Nuclear extracts from splenic lymphocytes from estrogen or placebo-treated mice which were Con-A-stimulated for 3, 6, 18, or 24 hrs of culture were analyzed for T-bet protein expression with a Western blot assay. One representative experiment is shown above the graph. The mean relative densitometry data of T-bet protein expression from nuclear extracts from Con-A stimulated splenic lymphocytes from estrogen or placebo-treated mice are shown (placebo (P): n=4 mice, estrogen (E2): n=4 mice; *p<0.05). (B) IFN-γ protein levels in supernatants from Con-A-activated splenocytes cultured for 3, 6, 18, or 24 hrs from estrogen and placebo treated mice were analyzed with an ELISA (placebo: n=4 mice, estrogen: n=4 mice; *p<0.05). (C) T-bet expression is increased in anti-CD3 antibody-stimulated splenocytes from estrogen-treated mice. Nuclear extracts from anti-CD3 antibody (10 μg/ml) stimulated splenic lymphocytes or unstimulated (media only) cells from placebo or estrogen-treated mice incubated for 3 hrs were assayed to determine T-bet protein expression. The top portion of the figure shows one representative experiment (n=4 mice per placebo (P) or estrogen (E2)-treatment; *p<0.05). The bottom portion of the figure depicts the mean relative densitometry data for T-bet protein expression as detected with Western blot analysis. Data are presented as means with standard error bars.
Figure 3. IL-12 does not directly alter T-bet protein expression in activated splenocytes from estrogen-treated mice
Splenocytes from estrogen or placebo-treated mice were stimulated with Con-A (10 μg/ml), Con-A and rIL-12 (20 ng/ml), or Con-A and anti-IL-12 antibodies (3 μg/ml) for 3 hrs and the expression of T-bet protein in the nuclear extracts was determined. (A) One representative experiment of T-bet protein expression in cells stimulated with Con-A or Con-A and rIL-12 is shown in the top portion of the figure. The bottom portion shows the mean relative densitometry data of T-bet protein expression (placebo (P): n=6 mice, estrogen (E2): n=6 mice; **p<0.01). (B) One representative experiment of T-bet protein expression is shown in the top portion of the figure. The bottom portion shows the mean relative densitometry data of T-bet protein expression in nuclear extracts from Con-A or Con-A and anti-IL-12-antibodies-activated splenocytes from estrogen or placebo-treated mice (placebo: n=10 mice, estrogen: n=10 mice; **p<0.01). Data are presented as means with standard error bars.
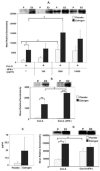
Figure 4. T-bet expression is partially regulated by IFN-γ in activated splenocytes and purified T cells from estrogen-treated mice
(A) Splenic lymphocytes from placebo or estrogen-treated mice were stimulated with Con-A (10 μg/ml) or Con-A plus various doses of rIFN-γ (100, 1,000, 10,000 pg/ml) for 3 hrs of culture and T-bet expression was explored in nuclear extracts of splenocytes. The top portion shows one representative experiment for T-bet expression. The figure demonstrates the mean relative densitometry data for T-bet protein expression (placebo: n=4 mice, estrogen: n=4 mice; *p<0.05). (B) Purified splenic T cells from estrogen or placebo-treated mice were cultured with Con-A (10 μg/ml) or Con-A and rIFN-γ (10,000 pg/ml) for 3 hrs and T-bet protein expression was explored in nuclear extracts from T cells (placebo: n=4 mice, estrogen: n=4 mice; **p<0.01). One representative experiment is shown at the top of Panel A. (C) IFN-γ protein levels in the supernatants from Con-A-activated T cells from estrogen and placebo-treated mice are shown (placebo: n=4 mice, estrogen: n=4 mice). (D) Intracellular IFN-γ protein in the cytoplasmic extracts from Con-A or Con-A and rIFN-γ-activated T cells was detected with Western blot assay. The mean relative densitometry data and one representative Western blot experiment are shown (placebo: n=4 mice, estrogen: n=4 mice; *p<0.05). Data are presented as means with standard error bars.
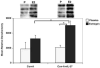
Figure 5. The early expression of T-bet is increased in response to deliberate addition of IL-27 to Con-A-activated splenocyte cultures from estrogen-treated mice
Nuclear extracts from Con-A (10 μg/ml), Con-A and rIL-27 (10 ng/ml) cultured splenic lymphocytes from placebo or estrogen-treated mice that were incubated for 3 hrs were assayed to determine T-bet expression. The top portion of the figure shows all 4 pairs of experiments (n=4 mice per placebo or estrogen-treatment). The bottom portion of the figure depicts the mean relative densitometry data for T-bet protein expression as detected with Western blot analysis (n=4 mice per placebo or estrogen-treatment; *p<0.05). Data are presented as means with standard error bars.
Similar articles
- Estrogen up-regulates inducible nitric oxide synthase, nitric oxide, and cyclooxygenase-2 in splenocytes activated with T cell stimulants: role of interferon-gamma.
Karpuzoglu E, Fenaux JB, Phillips RA, Lengi AJ, Elvinger F, Ansar Ahmed S. Karpuzoglu E, et al. Endocrinology. 2006 Feb;147(2):662-71. doi: 10.1210/en.2005-0829. Epub 2005 Nov 17. Endocrinology. 2006. PMID: 16293660 - Procyanidin B2 gallates inhibit IFN-γ and IL-17 production in T cells by suppressing T-bet and RORγt expression.
Tanaka S, Furuya K, Yamamoto K, Yamada K, Ichikawa M, Suda M, Makabe H. Tanaka S, et al. Int Immunopharmacol. 2017 Mar;44:87-96. doi: 10.1016/j.intimp.2017.01.007. Epub 2017 Jan 13. Int Immunopharmacol. 2017. PMID: 28088699 - IL-28A is a key regulator of T-cell-mediated liver injury via the T-box transcription factor T-bet.
Siebler J, Wirtz S, Weigmann B, Atreya I, Schmitt E, Kreft A, Galle PR, Neurath MF. Siebler J, et al. Gastroenterology. 2007 Jan;132(1):358-71. doi: 10.1053/j.gastro.2006.10.028. Epub 2006 Oct 21. Gastroenterology. 2007. PMID: 17241885 - Further checkpoints in Th1 development.
Robinson DS, O'Garra A. Robinson DS, et al. Immunity. 2002 Jun;16(6):755-8. doi: 10.1016/s1074-7613(02)00331-x. Immunity. 2002. PMID: 12121657 Review. - T-bet in disease.
Lazarevic V, Glimcher LH. Lazarevic V, et al. Nat Immunol. 2011 Jun 20;12(7):597-606. doi: 10.1038/ni.2059. Nat Immunol. 2011. PMID: 21685955 Free PMC article. Review.
Cited by
- Mechanisms underlying sex differences in autoimmunity.
Fairweather D, Beetler DJ, McCabe EJ, Lieberman SM. Fairweather D, et al. J Clin Invest. 2024 Sep 17;134(18):e180076. doi: 10.1172/JCI180076. J Clin Invest. 2024. PMID: 39286970 Free PMC article. Review. - Hallmarks of sex bias in immuno-oncology: mechanisms and therapeutic implications.
Xiao T, Lee J, Gauntner TD, Velegraki M, Lathia JD, Li Z. Xiao T, et al. Nat Rev Cancer. 2024 May;24(5):338-355. doi: 10.1038/s41568-024-00680-z. Epub 2024 Apr 8. Nat Rev Cancer. 2024. PMID: 38589557 - A viral-specific CD4+ T cell response protects female mice from Coxsackievirus B3 infection.
Pattnaik A, Dhalech AH, Condotta SA, Corn C, Richer MJ, Snell LM, Robinson CM. Pattnaik A, et al. Front Immunol. 2024 Jan 11;14:1327384. doi: 10.3389/fimmu.2023.1327384. eCollection 2023. Front Immunol. 2024. PMID: 38274806 Free PMC article. - Sex and Gender Differences in Tuberculosis Pathogenesis and Treatment Outcomes.
Dabitao D, Bishai WR. Dabitao D, et al. Curr Top Microbiol Immunol. 2023;441:139-183. doi: 10.1007/978-3-031-35139-6_6. Curr Top Microbiol Immunol. 2023. PMID: 37695428 Review. - Synthetically glycosylated antigens for the antigen-specific suppression of established immune responses.
Tremain AC, Wallace RP, Lorentz KM, Thornley TB, Antane JT, Raczy MR, Reda JW, Alpar AT, Slezak AJ, Watkins EA, Maulloo CD, Budina E, Solanki A, Nguyen M, Bischoff DJ, Harrington JL, Mishra R, Conley GP, Marlin R, Dereuddre-Bosquet N, Gallouët AS, LeGrand R, Wilson DS, Kontos S, Hubbell JA. Tremain AC, et al. Nat Biomed Eng. 2023 Sep;7(9):1142-1155. doi: 10.1038/s41551-023-01086-2. Epub 2023 Sep 7. Nat Biomed Eng. 2023. PMID: 37679570
References
- Afkarian M, Sedy JR, Yang J, Jacobson NG, Cereb N, Yang SY, Murphy TL, Murphy KM. T-bet is a STAT1-induced regulator of IL-12R expression in naive CD4+ T cells. Nat Immunol. 2002;3:549–57. - PubMed
- Ansar Ahmed S, Karpuzoglu-Sahin E. Estrogen, Interferon-gamma and Lupus. In: Zouali M, editor. Molecular Autoimmunity. Kluwer Academic/Plenum Publishers; New York: 2005.
- Chen Q, Ghilardi N, Wang H, Baker T, Xie MH, Gurney A, Grewal IS, de Sauvage FJ. Development of Th1-type immune responses requires the type I cytokine receptor TCCR. Nature. 2000;407:916–20. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials