A novel somatic mouse model to survey tumorigenic potential applied to the Hedgehog pathway - PubMed (original) (raw)
A novel somatic mouse model to survey tumorigenic potential applied to the Hedgehog pathway
Junhao Mao et al. Cancer Res. 2006.
Abstract
We report a novel mouse model for the generation of sporadic tumors and show the efficiency of this approach by surveying Hedgehog (Hh)-related tumors. Up-regulation of the Hh pathway is achieved by conditionally regulated expression of an activated allele of Smoothened (R26-SmoM2) using either sporadic leakage or global postnatal induction of a ubiquitously expressed inducible Cre transgene (CAGGS-CreER). Following postnatal tamoxifen induction, CAGGS-CreER; R26-SmoM2 mice developed tumors with short latency and high penetrance. All mice exhibited rhabdomyosarcoma and basal cell carcinoma; 40% also developed medulloblastoma. In addition, mice showed a novel pancreatic lesion resembling low-grade mucinous cystic neoplasms in humans. In contrast, widespread activation of SmoM2 in the postnatal prostate epithelium results in no detectable morphologic outcome in 12-month-old mice. Comparison of gene expression profiles among diverse tumors identified several signature genes, including components of platelet-derived growth factor and insulin-like growth factor pathways, which may provide a common mechanistic link to the Hh-related malignancies. This experimental model provides a robust tool for exploring the process of Hh-dependent tumorigenesis and the treatment of such tumors. More generally, this approach provides a genetic platform for identifying tumorigenic potential in putative oncogenes and tumor suppressors and for more effective modeling of sporadic cancers in mice.
Figures
Figure 1
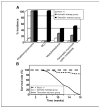
A somatic mouse model of Hh-related tumorigenesis. A, schematic representation of the CAGGS-CreER transgene and SmoM2 Rosa26 targeted alleles in the CAGGS-CreER; R26-SmoM2 model. B, recombination-mediated activation of SmoM2 by sporadic leakage and tamoxifen induction.
Figure 2
Tumor fomation in CAGGS-CreER; R26-SmoM2 mice. A, distinct tumor spectra in CAGGS-CreER; R26-moM2 mice. Histograms show the fraction of Ptch1+/− (white), CAGGS-CreER; R26-SmoM2 sporadic leakage group (blue), and tamoxifen postnatal injection group (red) mice that developed the indicated tumors. B, survival curves of Ptch1+/−, sporadic leakage group of CAGGS-CreER; R26-SmoM2, and tamoxifen postnatal injection group showing the fraction of mice that survive up to 18 weeks. Genotypes are color coded.
Figure 3
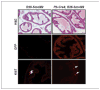
Rhabdomyosarcoma (RBS), BCC, and medulloblastoma in CAGGS-CreER; R26-SmoM2 mice. A, characterization of rhabdomyosarcoma from a CAGGS-CreER; R26-SmoM2 mouse. The muscle tumor shows a mixture of round undifferentiated cells and elongated spindle-shape cells. Desmin is present in both undifferentiated and differentiated tumor cells. B, BCC in tail skin of mice in the postnatal tamoxifen injection group. C, sporadic and tamoxifen-induced SmoM2 expression in the cerebellum induces medulloblastoma. Typical histologic features of human classic medulloblastoma with small blue cells, numerous mitoses, and little histologic evidence of differentiation. Medulloblastomas in CAGGS-CreER; R26-SmoM2 mice expressed Zic1, an early marker of neuronal differentiation, and NeuN, a later marker of neuronal differentiation (C). Ki67 staining indicates a high mitotic index in tumors relative to the WT tissues (A, B, and C). Immunostaining using an anti-GFP antibody shows expression of SmoM2-YFP in tumor cells (A, B, and C).
Figure 4
Pancreatic mucinous cystic lesion in CAGGS-CreER; R26-SmoM2 mice. Alcian blue and PAS staining shows focal intestinal-type mucin expression (arrows) in the epithelium of the cysts, but not in normal pancreatic tissues. Note prominent mucin expression in smaller cysts.
Figure 5
SmoM2 activation in postnatal prostate epithelium is not sufficient to induce neoplastic transformation. H&E staining was done on dorsal prostate from a WT R26-SmoM2 mouse and a Pb-Cre4; R26-SmoM2 mouse at 12 months of age. GFP antibody staining shows SmoM2-YFP expression in prostate epithelium. Ki67 staining of proliferative cells (white arrows) does not reveal a higher mitotic index in the prostate of Pb-Cre4; R26-SmoM2 mice compared with a WT control.
Figure 6
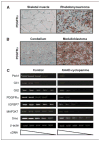
Regulation of the Hh, PDGFRα, IGF, and MAPK pathways in Hh-related tumors. Immunohistochemistry using an anti-PDGFRα antibody identifies strong PDGFRα staining in rhabdomyosarcomas (A) and medulloblastomas (B), relative to control skeletal muscle and cerebellar tissues. C, semiquantitative RT-PCR analysis of Hh-regulated mRNA expression in cultured primary medulloblastoma cells from CAGGS-CreER; R26-SmoM2 mice in the presence or absence of the SmoM2 inhibitor, KAAD-cyclopamine. Treatments were done for 72 hours. Descending wedge, serial dilutions of cDNA templates.
Similar articles
- Zfx facilitates tumorigenesis caused by activation of the Hedgehog pathway.
Palmer CJ, Galan-Caridad JM, Weisberg SP, Lei L, Esquilin JM, Croft GF, Wainwright B, Canoll P, Owens DM, Reizis B. Palmer CJ, et al. Cancer Res. 2014 Oct 15;74(20):5914-24. doi: 10.1158/0008-5472.CAN-14-0834. Epub 2014 Aug 27. Cancer Res. 2014. PMID: 25164012 Free PMC article. - INTU is essential for oncogenic Hh signaling through regulating primary cilia formation in basal cell carcinoma.
Yang N, Leung EL, Liu C, Li L, Eguether T, Jun Yao XJ, Jones EC, Norris DA, Liu A, Clark RA, Roop DR, Pazour GJ, Shroyer KR, Chen J. Yang N, et al. Oncogene. 2017 Aug 31;36(35):4997-5005. doi: 10.1038/onc.2017.117. Epub 2017 May 1. Oncogene. 2017. PMID: 28459465 Free PMC article. - Hedgehog beyond medulloblastoma and basal cell carcinoma.
Teglund S, Toftgård R. Teglund S, et al. Biochim Biophys Acta. 2010 Apr;1805(2):181-208. doi: 10.1016/j.bbcan.2010.01.003. Epub 2010 Jan 18. Biochim Biophys Acta. 2010. PMID: 20085802 Review. - Sustained Hedgehog signaling is required for basal cell carcinoma proliferation and survival: conditional skin tumorigenesis recapitulates the hair growth cycle.
Hutchin ME, Kariapper MS, Grachtchouk M, Wang A, Wei L, Cummings D, Liu J, Michael LE, Glick A, Dlugosz AA. Hutchin ME, et al. Genes Dev. 2005 Jan 15;19(2):214-23. doi: 10.1101/gad.1258705. Epub 2004 Dec 29. Genes Dev. 2005. PMID: 15625189 Free PMC article. - Advances in the treatment of Basal cell carcinoma: Hedgehog inhibitors.
Kudchadkar R, Lewis K, Gonzalez R. Kudchadkar R, et al. Semin Oncol. 2012 Apr;39(2):139-44. doi: 10.1053/j.seminoncol.2012.01.011. Semin Oncol. 2012. PMID: 22484185 Review.
Cited by
- Genome-wide CRISPR-Cas9 knockout screens identify DNMT1 as a druggable dependency in sonic hedgehog medulloblastoma.
Tsiami F, Lago C, Pozza N, Piccioni F, Zhao X, Lülsberg F, Root DE, Tiberi L, Kool M, Schittenhelm J, Bandopadhayay P, Segal RA, Tabatabai G, Merk DJ. Tsiami F, et al. Acta Neuropathol Commun. 2024 Aug 7;12(1):125. doi: 10.1186/s40478-024-01831-x. Acta Neuropathol Commun. 2024. PMID: 39107797 Free PMC article. - Childhood cancer mutagenesis caused by transposase-derived PGBD5.
Yamada M, Keller RR, Gutierrez RL, Cameron D, Suzuki H, Sanghrajka R, Vaynshteyn J, Gerwin J, Maura F, Hooper W, Shah M, Robine N, Demarest P, Bayin NS, Zapater LJ, Reed C, Hébert S, Masilionis I, Chaligne R, Socci ND, Taylor MD, Kleinman CL, Joyner AL, Raju GP, Kentsis A. Yamada M, et al. Sci Adv. 2024 Mar 22;10(12):eadn4649. doi: 10.1126/sciadv.adn4649. Epub 2024 Mar 22. Sci Adv. 2024. PMID: 38517960 Free PMC article. - Heterogeneity and tumoral origin of medulloblastoma in the single-cell era.
Sheng H, Li H, Zeng H, Zhang B, Lu Y, Liu X, Xu Z, Zhang J, Zhang L. Sheng H, et al. Oncogene. 2024 Mar;43(12):839-850. doi: 10.1038/s41388-024-02967-9. Epub 2024 Feb 14. Oncogene. 2024. PMID: 38355808 Free PMC article. Review. - Chronic AMPK inactivation slows SHH medulloblastoma progression by inhibiting mTORC1 signaling and depleting tumor stem cells.
Malawsky DS, Dismuke T, Liu H, Castellino E, Brenman J, Dasgupta B, Tikunov A, Gershon TR. Malawsky DS, et al. iScience. 2023 Nov 14;26(12):108443. doi: 10.1016/j.isci.2023.108443. eCollection 2023 Dec 15. iScience. 2023. PMID: 38094249 Free PMC article. - Single-fraction Radiation Treatment Dose Response in a Genetically Engineered Mouse Model of Medulloblastoma.
Tu KJ, Stewart CE, Williams NT, Ma Y, Luo L, Ghosh D, Weidenhammer LB, Floyd SR, Fan Y, Kirsch DG, Oldham M, Reitman ZJ. Tu KJ, et al. Radiat Res. 2023 Dec 1;200(6):587-592. doi: 10.1667/RADE-23-00126.1. Radiat Res. 2023. PMID: 37990957 Free PMC article.
References
- McMahon AP, Ingham PW, Tabin CJ. Developmental roles and clinical significance of hedgehog signaling. Curr Top Dev Biol. 2003;53:1–114. - PubMed
- Lum L, Beachy PA. The Hedgehog response network: sensors, switches, and routers. Science. 2004;304:1755–9. - PubMed
- Gorlin RJ. Nevoid basal-cell carcinoma syndrome. Medicine (Baltimore) 1987;66:98–113. - PubMed
- Johnson RL, Rothman AL, Xie J, et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272:1668–71. - PubMed
- Hahn H, Wicking C, Zaphiropoulous PG, et al. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell. 1996;85:841–51. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases