Myeloid cell trafficking and tumor angiogenesis - PubMed (original) (raw)
Review
Myeloid cell trafficking and tumor angiogenesis
Michael C Schmid et al. Cancer Lett. 2007.
Abstract
Tumor growth and metastasis depend on neovascularization, the growth of new blood vessels. Recent findings have revealed that tumor neovascularization is regulated in part by monocytes, which are myeloid lineage cells from the bone marrow. Tumors exhibit significant monocyte infiltrates, which are actively recruited to the tumor microenvironment. Upon tumor infiltration, monocytes can participate in tumor neovascularization. Monocytes can either differentiate into macrophages, which express proangiogenic growth factors, or into endothelial-like cells, which may directly participate in neovascularization. Preliminary studies in animals suggest that modulation of bone marrow-derived cell trafficking into tumors will provide a useful new approach in cancer therapy.
Figures
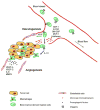
Figure 1. Bone marrow derived myeloid cells contribute to tumor angiogenesis
Myeloid precursor cells adhere to angiogenic endothelium via activated α4β1 or β2 integrins. Stromal derived growth factor 1 (SDF-1), colony stimulating factors (GM-CSF and others) or β-defensin mobilize specific subsets of myeloid precursor cells. In the presence of specific cytokines and growth factors, these cells differentiate either into endothelial-like cells, which are directly incorporated into new blood vessels (green arrow), or into monocytes / macrophages, which support tumor growth in a indirect manner (blue arrow). While interleukin-10 promotes differentiation of monocytes into M2 macrophages, VEGF and other factors promote endothelial cell differentiation from progenitor cells.
Similar articles
- Chapter 15. Methods to study myeloid cell roles in angiogenesis.
Schmid MC, Varner JA. Schmid MC, et al. Methods Enzymol. 2008;445:343-71. doi: 10.1016/S0076-6879(08)03015-2. Methods Enzymol. 2008. PMID: 19022067 - Influence of bone marrow-derived hematopoietic cells on the tumor response to radiotherapy: experimental models and clinical perspectives.
Ahn GO, Brown JM. Ahn GO, et al. Cell Cycle. 2009 Apr 1;8(7):970-6. doi: 10.4161/cc.8.7.8075. Epub 2009 Apr 4. Cell Cycle. 2009. PMID: 19270527 Free PMC article. Review. - Bone marrow derived cells in tumor angiogenesis and growth: are they the good, the bad or the evil?
Shaked Y, Voest EE. Shaked Y, et al. Biochim Biophys Acta. 2009 Aug;1796(1):1-4. doi: 10.1016/j.bbcan.2009.07.002. Biochim Biophys Acta. 2009. PMID: 19703646 - Role of myeloid cells in vascular endothelial growth factor-independent tumor angiogenesis.
Ferrara N. Ferrara N. Curr Opin Hematol. 2010 May;17(3):219-24. doi: 10.1097/MOH.0b013e3283386660. Curr Opin Hematol. 2010. PMID: 20308892 Review. - Myeloid cells functioning in tumor vascularization as a novel therapeutic target.
McLean K, Buckanovich RJ. McLean K, et al. Transl Res. 2008 Feb;151(2):59-67. doi: 10.1016/j.trsl.2007.11.002. Epub 2007 Dec 26. Transl Res. 2008. PMID: 18201673 Review.
Cited by
- Estrogen Promotes Resistance to Bevacizumab in Murine Models of NSCLC.
Patel SA, Herynk MH, Cascone T, Saigal B, Nilsson MB, Tran H, Ramachandran S, Diao L, Wang J, Le X, Minna J, Wistuba II, Heymach JV. Patel SA, et al. J Thorac Oncol. 2021 Dec;16(12):2051-2064. doi: 10.1016/j.jtho.2021.07.007. Epub 2021 Jul 24. J Thorac Oncol. 2021. PMID: 34311109 Free PMC article. - Role of bFGF in Acquired Resistance upon Anti-VEGF Therapy in Cancer.
Zahra FT, Sajib MS, Mikelis CM. Zahra FT, et al. Cancers (Basel). 2021 Mar 20;13(6):1422. doi: 10.3390/cancers13061422. Cancers (Basel). 2021. PMID: 33804681 Free PMC article. Review. - Targeting Myeloid-Derived Suppressor Cell, a Promising Strategy to Overcome Resistance to Immune Checkpoint Inhibitors.
Hou A, Hou K, Huang Q, Lei Y, Chen W. Hou A, et al. Front Immunol. 2020 May 15;11:783. doi: 10.3389/fimmu.2020.00783. eCollection 2020. Front Immunol. 2020. PMID: 32508809 Free PMC article. Review. - CCL25 promotes the migration and invasion of non-small cell lung cancer cells by regulating VEGF and MMPs in a CCR9-dependent manner.
Niu Y, Tang D, Fan L, Gao W, Lin H. Niu Y, et al. Exp Ther Med. 2020 Jun;19(6):3571-3580. doi: 10.3892/etm.2020.8635. Epub 2020 Apr 2. Exp Ther Med. 2020. PMID: 32346420 Free PMC article. - Anti-HER2 induced myeloid cell alterations correspond with increasing vascular maturation in a murine model of HER2+ breast cancer.
Bloom MJ, Jarrett AM, Triplett TA, Syed AK, Davis T, Yankeelov TE, Sorace AG. Bloom MJ, et al. BMC Cancer. 2020 Apr 28;20(1):359. doi: 10.1186/s12885-020-06868-4. BMC Cancer. 2020. PMID: 32345237 Free PMC article.
References
- Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;428:932–936. - PubMed
- Coultas L, Chawengsaksophak K, Rossant J. Endothelial cells and VEGF in vascular development. Nature. 2005;438:927–945. - PubMed
- Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;11:1194–1201. - PubMed
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;5302:964–967. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA083133/CA/NCI NIH HHS/United States
- CA83133/CA/NCI NIH HHS/United States
- R01 CA126820/CA/NCI NIH HHS/United States
- R01 CA083133-10/CA/NCI NIH HHS/United States
- R01 CA083133-11/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources