A common mechanism underlies stretch activation and receptor activation of TRPC6 channels - PubMed (original) (raw)
A common mechanism underlies stretch activation and receptor activation of TRPC6 channels
Maria A Spassova et al. Proc Natl Acad Sci U S A. 2006.
Abstract
The TRP family of ion channels transduce an extensive range of chemical and physical signals. TRPC6 is a receptor-activated nonselective cation channel expressed widely in vascular smooth muscle and other cell types. We report here that TRPC6 is also a sensor of mechanically and osmotically induced membrane stretch. Pressure-induced activation of TRPC6 was independent of phospholipase C. The stretch responses were blocked by the tarantula peptide, GsMTx-4, known to specifically inhibit mechanosensitive channels by modifying the external lipid-channel boundary. The GsMTx-4 peptide also blocked the activation of TRPC6 channels by either receptor-induced PLC activation or by direct application of diacylglycerol. The effects of the peptide on both stretch- and diacylglycerol-mediated TRPC6 activation indicate that the mechanical and chemical lipid sensing by the channel has a common molecular mechanism that may involve lateral-lipid tension. The mechanosensing properties of TRPC6 channels highly expressed in smooth muscle cells are likely to play a key role in regulating myogenic tone in vascular tissue.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Hypoosmotic- and pressure-induced stretch activation of nonselective cation current through TRPC6 channels. Currents were measured in HEK293 cells inducibly expressing TRPC6 or in isolated patches from CHO cells transiently expressing TRPC6. (A) In HEK293 cells induced to express TRPC6, a current of ≈200 pA at 80 mV developed after exposure to hypoosmotic extracellular solution. Cl− was substituted with glutamate to prevent volume-regulating Cl− currents. Addition of the TRPC6 agonist OAG did not induce further current. (B) The I/V relationship at maximal activation reveals typical TRPC6-like outward rectification. (C) In uninduced HEK293 cells, hypoosmotic- or OAG-induced currents were drastically reduced. Capacitance change during hypoosmotic swelling showed linear time dependence (Inset), and the I/V curve (D) revealed much reduced amplitude and slight outward rectification. (E) Summary of maximal currents at +100 mV for control (n = 3) and TRPC6-expressing cells (n = 4). (F–K) CHO cells, transiently expressing TRPC6, were used to study responses to directly applied pressure pulses. Time course of negative pressure applied to inside-out membrane patches from CHO-TRPC6 cells (F). Time course of activation of outwardly rectifying current after pressure pulse (G). Each point represents average current recorded for 150 ms at 60 mV (red) and −60 mV (black). Time courses of negative pressure and currents at ±60 mV in control transfected cells are shown in H and I, respectively. (J) Time course of the variance of the current in G. (K). Variance-current dependence revealed a single-channel conductance of activated channels of 25 pS, typical for TRPC6.
Fig. 2.
Time course and phospholipase C-independence of stretch-induced TRPC6 channel activation in CHO cells. (A–F) The PLC inhibitor U73122 was used to block possible activation of TRPC6 by PLC-linked stretch-activated receptors. TRPC6 was activated by a short (≈2 sec) pressure pulse (A) applied to inside-out membrane patches after >2 min of exposure to 10 μM U73122. (B) Average current at −60 mV (black) and +60 mV (red). (C) Actual current traces at −60 and +60 mV corresponding to indicated time points in B, that is, either 7 s prior (−7s), during (0 s), or 7 s after (+7 s) the pressure pulse. (D) Current–voltage dependence of the maximally activated current in B recorded by applying a 50-ms voltage ramp from −100 to +100 mV. (E and F) Noise analysis of the currents from B at +60 and −60 mV, respectively, revealing single-channel conductance of 25 pS. (G) Ca2+ release in response 100 μM carbachol in HEK cells in the absence of extracellular Ca2+ measured by Fura-2 in the presence (red) and absence (black) of 10 μM U73122. (H–M) Stretch activation of TRPC6 channels after 40-min incubation of CHO cells in 40 μM cytochalasin D. (H–J) Stretch-activated current from inside-out patch of TRPC6 expressing CHO cells. The current has typical TRPC6 outward rectification and short open times. (H) Time course of pressure applied to the patch. (I) Parallel time course of channel activation. (J) High time-resolution current at maximal activation in I. (K–M) Stretch activation in control cells, showing activation of distinct inwardly rectifying channels with long open times. Usually only 1 or 2 channels were present. (K) Time course of pressure applied to inside-out patch. (L) Parallel time course of current activation in control cells. (M) High time-resolution current from L.
Fig. 3.
Activation of TRPC6 channels in response to stretch, phospholipase C, or direct application of diacylglycerol, is inhibited by the tarantula peptide, GsMTx-4. (A and B) Inhibition of the hypoosmotic response of TRPC6 by GsMTx-4. (A) Acute addition of 5 μM GsMTx-4 to the activated TRPC6 increases the rate of channel inactivation. Currents are shown at 80 mV. (B) I/V relationship of the currents in A at maximal activation. (C and D) Inhibition by GsMTx-4 of stretch-activated current in CHO cells overexpressing TRPC6. Pressure pulses applied to an inside-out patch (C) did not activate TRPC6 after 2-min exposure to 5 μM GsMTx-4 added to the pipette solution (D). (E) Summary of the stretch-activated currents in inside-out patches from CHO cells overexpressing TRPC6. The bar chart represents control CHO cells, TRPC6-overexpressing cells in the presence of 5 μM GsMTx-4, 20 μM U73122, 40 μM cytochalasin D, and TRPC6 P112Q-overexpressing cells. (F–M) Whole-cell recordings from CHO cells expressing TRPC6 in the absence or presence of 5 μM GsMTx-4 applied 20–40 min before the trace. Time course of TRPC6 activation by the purinergic receptor agonist UTP at −80 (black) and +80 (red) mV (F); UTP-induced TRPC6 activation was inhibited by GsMTx-4, and inhibition was reversed by washout (G); I/V relationship for maximal activation of TRPC6 channels in absence (black) and presence (red) of 5 μM GsMTx4 (H); average peak current at 100 mV in the absence (black) and presence (red) of GsMTx-4 (both cases, n = 3) (I); time-course of TRPC6 activation by 100 μM OAG (J); activation of TRPC6 by OAG is inhibited after incubation with 5 μM GsMTx-4 (K); examples of I/V relationship of maximal OAG-induced TRPC6 channel activation in the absence (black) and presence (red) of 5 μM GsMTx-4 (L); and average peak current at 100 mV in the absence (black) and presence (red,) of GsMTx-4 (n = 3 and 4, respectively) (M).
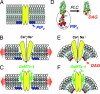
Fig. 4.
Model of TRPC6 activation by stretch and diacylglycerol and the inhibition of this activation by the tarantula peptide, GsMTx-4. (A) The resting closed state of the channel is depicted with PIP2 (blue head groups) in the inner leaflet vicinity of the channel. (B) Stretch of the cell membrane causes membrane thinning, inducing exposure and/or conformational change in the TRPC6 channel, resulting in its open state. (C) The GsMTx-4 peptide inserts in the outer membrane leaflet and, by modifying boundary lipids, relieves lipid stress and/or prevents channel exposure resulting in channel closure. (D) Receptor activation of phospholipase C results in cleavage of the large charged IP3 head group of PIP2 to produce the small uncharged head group on DAG. (E) The drastic change in lipid geometry on the inner leaflet in the vicinity of the channel creates membrane curvature, resulting in stress and/or exposure of the TRPC6 channel and its opening. (F) As with stretch-activation, the GsMTx-4 peptide inserts in the outer bilayer leaflet and, by modifying channel boundary lipids, relieves lipid stress and/or prevents channel exposure and inhibits channel gating.
Similar articles
- Synergistic activation of vascular TRPC6 channel by receptor and mechanical stimulation via phospholipase C/diacylglycerol and phospholipase A2/omega-hydroxylase/20-HETE pathways.
Inoue R, Jensen LJ, Jian Z, Shi J, Hai L, Lurie AI, Henriksen FH, Salomonsson M, Morita H, Kawarabayashi Y, Mori M, Mori Y, Ito Y. Inoue R, et al. Circ Res. 2009 Jun 19;104(12):1399-409. doi: 10.1161/CIRCRESAHA.108.193227. Epub 2009 May 14. Circ Res. 2009. PMID: 19443836 - A mutation in TRPC6 channels abolishes their activation by hypoosmotic stretch but does not affect activation by diacylglycerol or G protein signaling cascades.
Wilson C, Dryer SE. Wilson C, et al. Am J Physiol Renal Physiol. 2014 May 1;306(9):F1018-25. doi: 10.1152/ajprenal.00662.2013. Epub 2014 Mar 5. Am J Physiol Renal Physiol. 2014. PMID: 24598806 - Stretch-activation of angiotensin II type 1a receptors contributes to the myogenic response of mouse mesenteric and renal arteries.
Schleifenbaum J, Kassmann M, Szijártó IA, Hercule HC, Tano JY, Weinert S, Heidenreich M, Pathan AR, Anistan YM, Alenina N, Rusch NJ, Bader M, Jentsch TJ, Gollasch M. Schleifenbaum J, et al. Circ Res. 2014 Jul 7;115(2):263-72. doi: 10.1161/CIRCRESAHA.115.302882. Epub 2014 May 16. Circ Res. 2014. PMID: 24838176 - Role of TRPC3 and TRPC6 channels in the myocardial response to stretch: Linking physiology and pathophysiology.
Yamaguchi Y, Iribe G, Nishida M, Naruse K. Yamaguchi Y, et al. Prog Biophys Mol Biol. 2017 Nov;130(Pt B):264-272. doi: 10.1016/j.pbiomolbio.2017.06.010. Epub 2017 Jun 20. Prog Biophys Mol Biol. 2017. PMID: 28645743 Review. - TRPC6.
Dietrich A, Gudermann T. Dietrich A, et al. Handb Exp Pharmacol. 2007;(179):125-41. doi: 10.1007/978-3-540-34891-7_7. Handb Exp Pharmacol. 2007. PMID: 17217054 Review.
Cited by
- Inhibition and potentiation of the exercise pressor reflex by pharmacological modulation of TRPC6 in male rats.
Ducrocq GP, Anselmi L, Stella SL Jr, Copp SW, Ruiz-Velasco V, Kaufman MP. Ducrocq GP, et al. J Physiol. 2024 Feb 10:10.1113/JP286118. doi: 10.1113/JP286118. Online ahead of print. J Physiol. 2024. PMID: 38340081 - Revisiting TRPC1 and TRPC6 mechanosensitivity.
Gottlieb P, Folgering J, Maroto R, Raso A, Wood TG, Kurosky A, Bowman C, Bichet D, Patel A, Sachs F, Martinac B, Hamill OP, Honoré E. Gottlieb P, et al. Pflugers Arch. 2008 Mar;455(6):1097-103. doi: 10.1007/s00424-007-0359-3. Epub 2007 Oct 23. Pflugers Arch. 2008. PMID: 17957383 - Remembering Mechanosensitivity of NMDA Receptors.
Johnson LR, Battle AR, Martinac B. Johnson LR, et al. Front Cell Neurosci. 2019 Dec 5;13:533. doi: 10.3389/fncel.2019.00533. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31866826 Free PMC article. Review. - Transcellular calcium transport in mammary epithelial cells.
VanHouten JN, Wysolmerski JJ. VanHouten JN, et al. J Mammary Gland Biol Neoplasia. 2007 Dec;12(4):223-35. doi: 10.1007/s10911-007-9057-1. Epub 2007 Nov 13. J Mammary Gland Biol Neoplasia. 2007. PMID: 17999165 - Ca2+ release from the sarcoplasmic reticulum is required for sustained TRPM4 activity in cerebral artery smooth muscle cells.
Gonzales AL, Amberg GC, Earley S. Gonzales AL, et al. Am J Physiol Cell Physiol. 2010 Aug;299(2):C279-88. doi: 10.1152/ajpcell.00550.2009. Epub 2010 Apr 28. Am J Physiol Cell Physiol. 2010. PMID: 20427713 Free PMC article.
References
- Ramsey IS, Delling M, Clapham DE. Annu Rev Physiol. 2006;68:619–647. - PubMed
- Montell C. Sci STKE 2005. 2005:re3. - PubMed
- Putney JW. Pflugers Arch. 2005;451:29–34. - PubMed
- Venkatachalam K, van Rossum DB, Patterson RL, Ma HT, Gill DL. Nat Cell Biol. 2002;4:E263–E272. - PubMed
- Xu SZ, Beech DJ. Circ Res. 2001;88:84–87. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources