Sustained regression of tumors upon MYC inactivation requires p53 or thrombospondin-1 to reverse the angiogenic switch - PubMed (original) (raw)
. 2006 Oct 31;103(44):16266-71.
doi: 10.1073/pnas.0608017103. Epub 2006 Oct 20.
Sandra Ryeom, Alice C Fan, Pavan Bachireddy, Ryan C Lynch, Matthew J Rioth, Jan van Riggelen, Andrew M Kopelman, Emmanuelle Passegué, Flora Tang, Judah Folkman, Dean W Felsher
Affiliations
- PMID: 17056717
- PMCID: PMC1637571
- DOI: 10.1073/pnas.0608017103
Sustained regression of tumors upon MYC inactivation requires p53 or thrombospondin-1 to reverse the angiogenic switch
Sylvie Giuriato et al. Proc Natl Acad Sci U S A. 2006.
Abstract
The targeted inactivation of oncogenes offers a rational therapeutic approach for the treatment of cancer. However, the therapeutic inactivation of a single oncogene has been associated with tumor recurrence. Therefore, it is necessary to develop strategies to override mechanisms of tumor escape from oncogene dependence. We report here that the targeted inactivation of MYC is sufficient to induce sustained regression of hematopoietic tumors in transgenic mice, except in tumors that had lost p53 function. p53 negative tumors were unable to be completely eliminated, as demonstrated by the kinetics of tumor cell elimination revealed by bioluminescence imaging. Histological examination revealed that upon MYC inactivation, the loss of p53 led to a deficiency in thrombospondin-1 (TSP-1) expression, a potent antiangiogenic protein, and the subsequent inability to shut off angiogenesis. Restoration of p53 expression in these tumors re-established TSP-1 expression. This permitted the suppression of angiogenesis and subsequent sustained tumor regression upon MYC inactivation. Similarly, the restoration of TSP-1 alone in p53 negative tumors resulted in the shut down of angiogenesis and led to sustained tumor regression upon MYC inactivation. Hence, the complete regression of tumor mass driven by inactivation of the MYC oncogene requires the p53-dependent induction of TSP-1 and the shut down of angiogenesis. Notably, overexpression of TSP-1 alone did not influence tumor growth. Therefore, the combined inactivation of oncogenes and angiogenesis may be a more clinically effective treatment of cancer. We conclude that angiogenesis is an essential component of oncogene addiction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
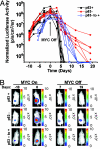
Brief MYC inactivation can induce sustained tumor regression. (A) Kaplan–Meier plots for tumor-free survival after doxycycline treatment (100 μg/ml). The genotypes of the mice and the sustained (MYC Off) or brief (MYC Off/On) MYC inactivation are indicated. The number of mice in each group is denoted by the n values. Primary tumor regression and relapse were monitored for up to 50 weeks. Statistical comparison of Kaplan–Meier plots is based on the log-rank test. (B) p53 protein expression levels in MYC primary and relapsed tumors. Western blot analysis was performed on protein lysates prepared from MYC primary and relapsed tumors. Equal loading was confirmed by anti-α tubulin Western blot analysis. PC, positive control. (C) p53 protein and mRNA expression in primary (10) MYC versus primary MYC/p53+/− tumors. PCR was performed to measure p53 and gapdh mRNA. Western blot analysis of p53, MYC, and tubulin protein expression were performed.
Fig. 2.
p53 status and the rate of tumor cell elimination upon MYC inactivation. (A) Measurement of tumor cell elimination upon MYC inactivation. p53 wild-type (p53+), p53 negative (p53−), and p53 restored (p53− to +) tumor cells were retrovirally labeled with luciferase before s.c. injection into syngeneic mice. Mice were serially imaged by bioluminescence imaging at the indicated times, before and after MYC inactivation, to monitor transplanted tumor growth (MYC On) and tumor regression upon doxycycline treatment (100 μg/ml) (MYC Off). A summary of changes in luciferase activity is shown. Each cohort consisted of at least 5 mice. Representative data from one of three experiments is shown. (B) Representative images of bioluminescence imaging.
Fig. 3.
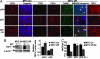
p53 status modifies the angiogenic response of tumors upon MYC inactivation. (A Left) p53 status does not affect the angiogenic response of tumor onset upon MYC activation. p53 wild-type (p53+), p53 negative (p53−) and p53 restored (p53− to +) transplanted tumors were examined by immunofluorescence for microvessel density (CD31) and TSP-1 expression (MYC on). (A Right) The loss of p53 decreases TSP-1 levels despite MYC inactivation. p53 wild-type (p53+), p53 negative and p53 restored (p53− to +) transplanted tumors were examined by immunofluorescence for microvessel density (CD31) and TSP-1 expression 6 days after MYC inactivation (MYC Off). Whereas there was no significant difference in the level of TUNEL reactivity, there were significantly more TUNEL-positive endothelial cells (white arrow) in p53 wild-type (p53+) and p53 restored (p53− to +) tumors as compared with p53 negative (p53−) tumors. Specificity of staining was ensured by immunostaining of serial tumor sections with isotype matched control antibodies. (B) Western blot analysis of p53 wild-type (p53+) and p53 negative (p53−) transplanted tumors were performed for TSP-1 expression upon MYC activation and 6 days after MYC inactivation. Densitometric analysis demonstrated significantly higher TSP-1 expression in p53 wild-type (p53+) tumor cells relative to β-actin as compared with p53 negative (p53−) tumor cells. (C) Quantification of microvessel density (MVD) per high power field (hpf) demonstrated significantly increased MVD/hpf in p53 negative (p53−) tumors 6 days after MYC inactivation as compared with p53 wild-type (p53+) tumors. At least five fields were counted in representative tumor sections, and at least two different transplanted tumors were analyzed. Values are represented as means ± standard deviation.
Fig. 4.
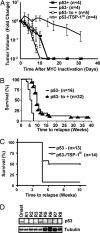
Combined inactivation of MYC and restoration of p53 or TSP-1 expression is more effective in inducing tumor regression. (A) Restoration of p53 or TSP-1 and tumor regression upon MYC inactivation. In these experiments, p53 or TSP-1 was transduced into tumor cells and maintained under continuous selection in vitro to ensure expression. p53 positive (p53+), p53 negative (p53−), p53 restored (p53− to +) and p53-/TSP-1HI tumor cells were injected s.c. at a dose of 107 cells into cohorts of syngeneic FVB/N-recipient mice. When transplanted tumors reached 2 cm2 in size, mice were treated with doxycycline (100 μg/ml) to inactivate MYC. Tumor volume is calculated from caliper measurement and is expressed as logarithmic fold changes in tumor volume before MYC inactivation. Data are presented as the mean for each cohort ± SEM with statistical analysis performed by the Mann–Whitney test. (B and C) MYC inactivation, combined with p53 or TSP-1 restoration, delays tumor relapse in vivo. p53 negative (p53−), p53 restored (p53− to +), or TSP-1 restored (p53-/TSP-1HI) cells (107) were injected i.p. into syngeneic mice. When moribund with tumor burden, transplanted mice were treated with doxycycline (100 μg/ml) and monitored for tumor relapse. Kaplan–Meier survival curve are represented. Statistical comparison of Kaplan–Meier plots is based on the log-rank test. The number of mice in each group is denoted by n values. (D) Tumor relapse after MYC inactivation is frequently associated with reduced p53 protein expression. p53 expression levels in p53 restored tumor cells, at the time of tumor onset and relapse (R1–R8), was analyzed by Western blot on protein lysates (60 μg) prepared from transplanted relapsed tumor tissues. Protein loading was assayed by anti-α-tubulin Western blot.
Similar articles
- p19ARF is a critical mediator of both cellular senescence and an innate immune response associated with MYC inactivation in mouse model of acute leukemia.
Yetil A, Anchang B, Gouw AM, Adam SJ, Zabuawala T, Parameswaran R, van Riggelen J, Plevritis S, Felsher DW. Yetil A, et al. Oncotarget. 2015 Feb 28;6(6):3563-77. doi: 10.18632/oncotarget.2969. Oncotarget. 2015. PMID: 25784651 Free PMC article. - The multi-step process of human skin carcinogenesis: a role for p53, cyclin D1, hTERT, p16, and TSP-1.
Burnworth B, Arendt S, Muffler S, Steinkraus V, Bröcker EB, Birek C, Hartschuh W, Jauch A, Boukamp P. Burnworth B, et al. Eur J Cell Biol. 2007 Dec;86(11-12):763-80. doi: 10.1016/j.ejcb.2006.11.002. Epub 2007 Jan 2. Eur J Cell Biol. 2007. PMID: 17198740 - Regulation of tumor angiogenesis by thrombospondin-1.
Ren B, Yee KO, Lawler J, Khosravi-Far R. Ren B, et al. Biochim Biophys Acta. 2006 Apr;1765(2):178-88. doi: 10.1016/j.bbcan.2005.11.002. Epub 2005 Dec 21. Biochim Biophys Acta. 2006. PMID: 16406676 Review. - Conditional transgenic models define how MYC initiates and maintains tumorigenesis.
Arvanitis C, Felsher DW. Arvanitis C, et al. Semin Cancer Biol. 2006 Aug;16(4):313-7. doi: 10.1016/j.semcancer.2006.07.012. Epub 2006 Jul 21. Semin Cancer Biol. 2006. PMID: 16935001 Review.
Cited by
- Systemic Akt1 Deletion after Tumor Onset in p53(-/-) Mice Increases Lifespan and Regresses Thymic Lymphoma Emulating p53 Restoration.
Yu WN, Nogueira V, Sobhakumari A, Patra KC, Bhaskar PT, Hay N. Yu WN, et al. Cell Rep. 2015 Jul 28;12(4):610-21. doi: 10.1016/j.celrep.2015.06.057. Epub 2015 Jul 16. Cell Rep. 2015. PMID: 26190111 Free PMC article. - Cancer prevention as biomodulation: targeting the initiating stimulus and secondary adaptations.
Furth PA. Furth PA. Ann N Y Acad Sci. 2012 Oct;1271(1):1-9. doi: 10.1111/j.1749-6632.2012.06736.x. Ann N Y Acad Sci. 2012. PMID: 23050958 Free PMC article. Review. - Inhibition of Aurora A Kinase in Combination with Chemotherapy Induces Synthetic Lethality and Overcomes Chemoresistance in Myc-Overexpressing Lymphoma.
Park SI, Lin CP, Ren N, Angus SP, Dittmer DP, Foote M, Parton T, Bhatt AP, Fedoriw YD, Roth DP, Cann ML, Johnson GL, Damania B. Park SI, et al. Target Oncol. 2019 Oct;14(5):563-575. doi: 10.1007/s11523-019-00662-4. Target Oncol. 2019. PMID: 31429028 Free PMC article. - Oncogene withdrawal engages the immune system to induce sustained cancer regression.
Casey SC, Li Y, Fan AC, Felsher DW. Casey SC, et al. J Immunother Cancer. 2014 Jul 15;2:24. doi: 10.1186/2051-1426-2-24. eCollection 2014. J Immunother Cancer. 2014. PMID: 25089198 Free PMC article. Review. - Novel tissue-specific mechanism of regulation of angiogenesis and cancer growth in response to hyperglycemia.
Bhattacharyya S, Sul K, Krukovets I, Nestor C, Li J, Adognravi OS. Bhattacharyya S, et al. J Am Heart Assoc. 2012 Dec;1(6):e005967. doi: 10.1161/JAHA.112.005967. Epub 2012 Dec 19. J Am Heart Assoc. 2012. PMID: 23316333 Free PMC article.
References
- Bishop JM. Cell. 1991;64:235–248. - PubMed
- Hahn WC, Weinberg RA. N Engl J Med. 2002;347:1593–1603. - PubMed
- Sawyers CL. Cancer Cell. 2002;1:13–15. - PubMed
- Felsher DW. Nat Rev Cancer. 2003;3:375–379. - PubMed
- Giuriato S, Rabin K, Fan AC, Shachaf CM, Felsher DW. Semin Cancer Biol. 2004;14:3–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA 105102/CA/NCI NIH HHS/United States
- U56 CA112973/CA/NCI NIH HHS/United States
- 1P20 CA 112973/CA/NCI NIH HHS/United States
- R01 CA089305/CA/NCI NIH HHS/United States
- R01 CA105102/CA/NCI NIH HHS/United States
- 3R01 CA 089305-03S1/CA/NCI NIH HHS/United States
- R01 CA 85610/CA/NCI NIH HHS/United States
- R01 CA085610/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous