N-cadherin is an in vivo substrate for protein tyrosine phosphatase sigma (PTPsigma) and participates in PTPsigma-mediated inhibition of axon growth - PubMed (original) (raw)
N-cadherin is an in vivo substrate for protein tyrosine phosphatase sigma (PTPsigma) and participates in PTPsigma-mediated inhibition of axon growth
Roberta Siu et al. Mol Cell Biol. 2007 Jan.
Abstract
Protein tyrosine phosphatase sigma (PTPsigma) belongs to the LAR family of receptor tyrosine phosphatases and was previously shown to negatively regulate axon growth. The substrate for PTPsigma and the effector(s) mediating this inhibitory effect were unknown. Here we report the identification of N-cadherin as an in vivo substrate for PTPsigma. Using brain lysates from PTPsigma knockout mice, in combination with substrate trapping, we identified a hyper-tyrosine-phosphorylated protein of approximately 120 kDa in the knockout animals (relative to sibling controls), which was identified by mass spectrometry and immunoblotting as N-cadherin. beta-Catenin also precipitated in the complex and was also a substrate for PTPsigma. Dorsal root ganglion (DRG) neurons, which highly express endogenous N-cadherin and PTPsigma, exhibited a faster growth rate in the knockout mice than in the sibling controls when grown on laminin or N-cadherin substrata. However, when N-cadherin function was disrupted by an inhibitory peptide or lowering calcium concentrations, the differential growth rate between the knockout and sibling control mice was greatly diminished. These results suggest that the elevated tyrosine phosphorylation of N-cadherin in the PTPsigma(-/-) mice likely disrupted N-cadherin function, resulting in accelerated DRG nerve growth. We conclude that N-cadherin is a physiological substrate for PTPsigma and that N-cadherin (and likely beta-catenin) participates in PTPsigma-mediated inhibition of axon growth.
Figures
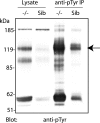
FIG. 1.
Brain lysates from PTPσ−/− mice contain several hyper-tyrosine-phosphorylated proteins. Equal amounts of protein from brain lysates of PTPσ knockout (−/−) mice and their sibling (Sib) controls (PTPσ+/+ and PTPσ+/−) were either separated by SDS-PAGE and immunoblotted with anti-pTyr antibodies or immunoprecipitated with anti-pTyr antibodies prior to immunoblotting with the same antibodies. The arrow indicates the protein band (∼120 kDa) that was cut out for further analysis by mass spectrometry and identified as N-cadherin.
FIG. 2.
N-cadherin is an in vivo and an in vitro substrate of PTPσ. (A and B) Immobilized PTPσ substrate trapping mutant GST-σD1(DA) and the corresponding wild type, GST-σD1(WT), as well as GST alone, were incubated with brain lysates from (A) sibling (Sib) controls or (B) PTPσ knockout (−/−) mice. Precipitated proteins were then separated by SDS-PAGE and immunoblotted with anti-pTyr or anti-N-cadherin (anti-N-cad) antibodies, as indicated. (C) Tyrosine-phosphorylated N-cad is detected in brain lysates of PTPσ knockout mice but not sibling controls. Brain lysates from PTPσ knockout mice or sibling controls were immunoprecipitated with anti-pTyr antibodies and immunoblotted with anti-N-cad or anti-pTyr antibodies. (D) Vanadate (VO4) reduces trapping of N-cad. Brain lysates from PTPσ−/− mice were incubated with the indicated trapping constructs in the presence or absence of the PTP inhibitor vanadate (10 mM), and precipitated proteins were separated by SDS-PAGE and immunoblotted with anti-N-cad antibodies. (E) N-cad is trapped with catalytically inactive first or both PTPase domains of PTPσ. HEK293T cells transfected with N-cad were lysed, lysates were incubated with the GST fusion protein, active (WT) or inactive (DA mutant), of the first (D1) or both (D1D2) catalytic domains of PTPσ, and precipitated proteins were immunoblotted for N-cad. Since the second domain (D2) is naturally inactive, only the first domain (D1) was inactivated using the DA mutation [GST-σD1(DA) and GSTσD1(DA)D2 represent the inactive first or both catalytic domains, respectively]. For panels A, B, D, and E, the lower parts of the panels depict Ponceau S staining of the blots, shown to verify that equal amounts of substrate trapping proteins were used for precipitating N-cad. (F) Ectopic expression of PTPσ in cells leads to dephosphorylation of endogenous N-cad. Flag-tagged PTPσ (full length, D1, or D1D2; all WT) were transfected into HEK293T cells, the cells were lysed, lysates were incubated with anti-pTyr antibodies, and precipitated proteins were immunoblotted with anti-N-cad antibodies to detect the extent of N-cad dephosphorylation. The lower panel shows the levels of expression of the indicated Flag-tagged proteins. tfxn, transfection. (G) PTPσ can directly dephosphorylate N-cad. N-cad overexpressed in HEK293T cells was immunopurified with N-cad antibodies and incubated (or not) with purified, GST-tagged PTPσD1(WT). Proteins were then immunoblotted with anti-pTyr antibodies to analyze the extent of N-cad dephosphorylation or with anti-N-cad antibodies to demonstrate equal amounts of N-cad precipitated in the experiment.
FIG. 3.
β-Catenin binds N-cadherin (N-cad) in vivo and is also a substrate for PTPσ. (A) Coimmunoprecipitation of N-cad with β-catenin (β-cat). Brain lysates from sibling (Sib) control or PTPσ−/− (−/−) mice were immunoprecipitated with anti-N-cad antibodies. The immunoprecipitates were separated by SDS-PAGE and immunoblotted with either anti-N-cad or anti-β-cat antibodies. (B) Substrate trapping of β-cat. PTPσ−/− brain lysates were incubated with immobilized GST or the PTPσ substrate trapping mutant GST-σD1(DA). The associated proteins were then analyzed by probing with antibodies against N-cad or β-cat. Ponceau S staining to visualize the amount of GST or GST-σD1(DA) substrate trapping proteins used is shown in the lower panel. (C and D) Hyper-tyrosine phosphorylation of β-cat in knockout mice. Brain lysates from Sib or PTPσ−/− mice were immunoprecipitated with anti-pTyr antibodies and immunoblotted for β-cat. For panel D, lysates were boiled in 6% SDS prior to the IP. (E) Vanadate (VO4) impairs substrate trapping of β-catenin. Brain lysates from PTPσ−/− mice were incubated with the indicated trapping constructs in the presence or absence of the PTP inhibitor vanadate (10 mM), and precipitated proteins were separated by SDS-PAGE and immunoblotted with anti-β-cat antibodies. Ponceau S staining to verify equal amounts of trapping proteins is shown in Fig. 2D. (F) Ectopic expression of PTPσ in cells leads to dephosphorylation of endogenous β-cat. Flag-tagged PTPσ constructs (full length, D1, or D1D2; all WT) were transfected into HEK293T cells, the cells were lysed, lysates were incubated with anti-pTyr antibodies, and precipitated proteins were immunoblotted with anti-β-cat antibodies to detect β-cat dephosphorylation. Levels of expression of the indicated Flag-tagged proteins are depicted in Fig. 2F. tfxn, transfection. (G) PTPσ can directly dephosphorylate β-cat. β-Catenin from HEK293T cells overexpressing N-cad was immunopurified with β-cat antibodies and incubated (or not) with purified, GST-tagged PTPσD1(WT). Proteins were then immunoblotted with anti-pTyr antibodies to analyze β-cat dephosphorylation or with anti-β-cat antibodies to demonstrate similar amounts of precipitated β-cat in the experiment.
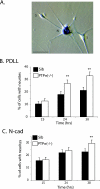
FIG. 4.
DRG neurons from PTPσ−/− mice exhibit a faster rate of neurite outgrowth. (A) LacZ staining of PTPσ−/− DRG culture, showing endogenous expression (blue) of PTPσ in the cell bodies of neurons (N) and, to a lesser extent, of Schwann cells (S). There was no LacZ staining seen in WT (+/+) DRG (not shown). Bar, 40 μm. (B and C) DRG cultures were prepared from sibling (Sib) controls and PTPσ−/− mice, and cells were plated on either (B) PDLL or (C) N-cadherin (N-cad). Neurite growth was measured at the indicated times. Data are expressed as the percentages of cells counted that have neurite extensions. A cell is counted positive if it has neurites greater than twice its body length. Data are summaries of seven independent experiments, and a total of at least 750 cells were counted for each condition. Error bars represent 95% confidence intervals. **, P < 0.0001 (chi-square test).
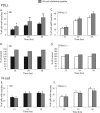
FIG. 5.
N-cadherin blocking peptide causes increased neurite outgrowth on PDLL of siblings but not PTPσ−/− neurons. DRG neurons from (A and B) sibling (Sib) controls or (C and D) PTPσ−/− mice were cultured on PDLL and treated with (gray bars) or without (black or white bars) 0.1 mg/ml of the N-cadherin (N-cad) inhibitory peptide INPISGQ. In panels A and C, data represent the percentages of cells counted that have neurite extensions at the indicated times, and these results are normalized to no-treatment controls in panels B and D, respectively. Data are summaries of four or five independent experiments, with a total of at least 500 cells counted for each condition. Error bars represent 95% confidence intervals. *, P < 0.001; **, P < 0.0001 (chi-square test). (E and F) As for panels A and C, only DRG were cultured on N-cad substratum. Data are summaries of four independent experiments, and a total of at least 500 cells were counted for each condition. Error bars represent 95% confidence intervals.
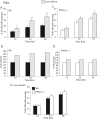
FIG. 6.
Low-calcium medium leads to increased neurite outgrowth on PDLL of siblings but not PTPσ−/− neurons. Sibling (Sib) control (A and B) and PTPσ−/− (C and D) DRG neurons grown on PDLL were cultured in normal neuronal medium (1.05 mM Ca2+) (black or white bars) or low-calcium medium (0.14 mM Ca2+) (gray bars). In panels B and D, data represent percentages of cells with neurite extensions normalized to those in panels A and C, respectively. (E). Neurite growth on PDLL in low-calcium medium (0.14 mM Ca2+) abolishes the difference in growth rate observed between the sibling control and PTPσ−/− neurons. Data are summaries of six independent experiments, and a total of at least 800 cells were counted for each condition. Error bars represent 95% confidence intervals. *, P < 0.001; **, P < 0.0001 (chi-square test).
FIG. 7.
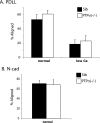
Alignment of Schwann cells to axons is not affected in the PTPσ−/− DRG. DRG cells from sibling (Sib) control and PTPσ−/− mice were plated on either (A) PDLL or (B) N-cadherin (N-cad). Alignment of SCs to axons was assessed after the cells were allowed to grow for (A) 40 h or (B) 46 h. An SC is counted positive for alignment when at least two of its processes are aligned with axons. Data represent means ± standard deviations of percentages of SCs aligned with axons from six independent experiments. A total of at least 700 cells were counted for each condition.
FIG. 8.
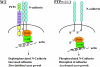
Model for PTPσ-regulated axon growth via the N-cadherin pathway. When PTPσ is present on the plasma membrane, it can dephosphorylate N-cadherin and recruit β-catenin (β-cat) to the complex. β-Catenin itself also becomes dephosphorylated, and this allows the association of the N-cadherin complex with the actin cytoskeleton, resulting in increased adhesion and reduced rate of axon growth. Conversely, when PTPσ is absent (e.g., in the knockout mice), N-cadherin and β-catenin remain phosphorylated on tyrosine residues. This prevents interaction between the two proteins, resulting in a loss of N-cadherin adhesive function and faster rate of axon growth. α-cat, α-catenin.
Similar articles
- Protein tyrosine phosphatase sigma-deficient mice show aberrant cytoarchitecture and structural abnormalities in the central nervous system.
Meathrel K, Adamek T, Batt J, Rotin D, Doering LC. Meathrel K, et al. J Neurosci Res. 2002 Oct 1;70(1):24-35. doi: 10.1002/jnr.10382. J Neurosci Res. 2002. PMID: 12237861 - Inhibition of N-cadherin and beta-catenin function reduces axon-induced Schwann cell proliferation.
Gess B, Halfter H, Kleffner I, Monje P, Athauda G, Wood PM, Young P, Wanner IB. Gess B, et al. J Neurosci Res. 2008 Mar;86(4):797-812. doi: 10.1002/jnr.21528. J Neurosci Res. 2008. PMID: 17941050 - Neuronal defects and posterior pituitary hypoplasia in mice lacking the receptor tyrosine phosphatase PTPsigma.
Wallace MJ, Batt J, Fladd CA, Henderson JT, Skarnes W, Rotin D. Wallace MJ, et al. Nat Genet. 1999 Mar;21(3):334-8. doi: 10.1038/6866. Nat Genet. 1999. PMID: 10080192 - The receptor tyrosine phosphatase CRYPalpha affects growth cone morphology.
Mueller BK, Ledig MM, Wahl S. Mueller BK, et al. J Neurobiol. 2000 Aug;44(2):204-18. J Neurobiol. 2000. PMID: 10934323 Review. - The regulation of cadherin-mediated adhesion by tyrosine phosphorylation/dephosphorylation of beta-catenin.
Lilien J, Balsamo J. Lilien J, et al. Curr Opin Cell Biol. 2005 Oct;17(5):459-65. doi: 10.1016/j.ceb.2005.08.009. Curr Opin Cell Biol. 2005. PMID: 16099633 Review.
Cited by
- Integrating virtual and biochemical screening for protein tyrosine phosphatase inhibitor discovery.
Martin KR, Narang P, Medina-Franco JL, Meurice N, MacKeigan JP. Martin KR, et al. Methods. 2014 Jan 15;65(2):219-28. doi: 10.1016/j.ymeth.2013.08.013. Epub 2013 Aug 20. Methods. 2014. PMID: 23969317 Free PMC article. - Protein tyrosine phosphatases PTPδ, PTPσ, and LAR: presynaptic hubs for synapse organization.
Takahashi H, Craig AM. Takahashi H, et al. Trends Neurosci. 2013 Sep;36(9):522-34. doi: 10.1016/j.tins.2013.06.002. Epub 2013 Jul 5. Trends Neurosci. 2013. PMID: 23835198 Free PMC article. Review. - Structural genomics of protein phosphatases.
Almo SC, Bonanno JB, Sauder JM, Emtage S, Dilorenzo TP, Malashkevich V, Wasserman SR, Swaminathan S, Eswaramoorthy S, Agarwal R, Kumaran D, Madegowda M, Ragumani S, Patskovsky Y, Alvarado J, Ramagopal UA, Faber-Barata J, Chance MR, Sali A, Fiser A, Zhang ZY, Lawrence DS, Burley SK. Almo SC, et al. J Struct Funct Genomics. 2007 Sep;8(2-3):121-40. doi: 10.1007/s10969-007-9036-1. Epub 2007 Dec 5. J Struct Funct Genomics. 2007. PMID: 18058037 Free PMC article. Review. - Receptor protein tyrosine phosphatases and cancer: new insights from structural biology.
Nikolaienko RM, Agyekum B, Bouyain S. Nikolaienko RM, et al. Cell Adh Migr. 2012 Jul-Aug;6(4):356-64. doi: 10.4161/cam.21242. Epub 2012 Jul 1. Cell Adh Migr. 2012. PMID: 22796942 Free PMC article. Review. - Investigation of protein-tyrosine phosphatase 1B function by quantitative proteomics.
Mertins P, Eberl HC, Renkawitz J, Olsen JV, Tremblay ML, Mann M, Ullrich A, Daub H. Mertins P, et al. Mol Cell Proteomics. 2008 Sep;7(9):1763-77. doi: 10.1074/mcp.M800196-MCP200. Epub 2008 May 31. Mol Cell Proteomics. 2008. PMID: 18515860 Free PMC article.
References
- Aberle, H., S. Butz, J. Stappert, H. Weissig, R. Kemler, and H. Hoschuetzky. 1994. Assembly of the cadherin-catenin complex in vitro with recombinant proteins. J. Cell Sci. 107:3655-3663. - PubMed
- Aberle, H., H. Schwartz, and R. Kemler. 1996. Cadherin-catenin complex: protein interactions and their implications for cadherin function. J. Cell. Biochem. 61:514-523. - PubMed
- Alonso, A., J. Sasin, N. Bottini, I. Friedberg, A. Osterman, A. Godzik, T. Hunter, J. Dixon, and T. Mustelin. 2004. Protein tyrosine phosphatases in the human genome. Cell 117:699-711. - PubMed
- Andersen, J. N., P. G. Jansen, S. M. Echwald, O. H. Mortensen, T. Fukada, R. Del Vecchio, N. K. Tonks, and N. P. Moller. 2004. A genomic perspective on protein tyrosine phosphatases: gene structure, pseudogenes, and genetic disease linkage. FASEB J. 18:8-30. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous