Reconstitution of protein targeting to the inner envelope membrane of chloroplasts - PubMed (original) (raw)
Reconstitution of protein targeting to the inner envelope membrane of chloroplasts
Ming Li et al. J Cell Biol. 2006.
Abstract
The chloroplast envelope plays critical roles in the synthesis and regulated transport of key metabolites, including intermediates in photosynthesis and lipid metabolism. Despite this importance, the biogenesis of the envelope membranes has not been investigated in detail. To identify the determinants of protein targeting to the inner envelope membrane (IM), we investigated the targeting of the nucleus-encoded integral IM protein, atTic40. We found that pre-atTic40 is imported into chloroplasts and processed to an intermediate size (int-atTic40) before insertion into the IM. Int-atTic40 is soluble and inserts into the IM from the internal stromal compartment. We also show that atTic40 and a second IM protein, atTic110, can target and insert into isolated IM vesicles in vitro. Collectively, our experiments are consistent with a "postimport" mechanism in which the IM proteins are first imported from the cytoplasm and subsequently inserted into the IM from the stroma.
Figures
Figure 1.
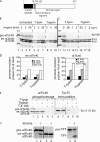
atTic40 targeting involves a size intermediate. (A) Schematic representation of the structure of pre-atTic40. The numbers indicate the positions of amino acid residues within the primary structure. (B) In vitro–translated [35S]pre-atTic40 was imported into isolated pea chloroplasts for the times indicated. Equivalent portions of each import reaction were treated in the absence (untreated) or presence of 100 μg/ml thermolysin (T-lysin) or 50 μg/ml trypsin to remove protein that was not fully imported. The total chloroplast fractions from each treatment and time point were analyzed by SDS-PAGE and phosphorimaging. (C) [35S]pre-atTic40 was imported into isolated pea chloroplasts for 5 min. Equivalent portions were treated with the indicated concentrations of thermolysin or trypsin. (D) Quantitative analysis of the levels of protease-resistant int-atTic40 and mature atTic40 from the import reactions in B. (E) Chloroplasts from a 5-min import reaction were treated with 200 μg/ml trypsin or thermolysin in the presence or absence of 1% Triton X-100 as indicated. The samples were analyzed by SDS-PAGE and phosphorimaging to detect imported [35S]pre-atTic40 (left) or immunoblotting to detect Toc75 (right). (F) Pre-atTic40 is processed to int-atTic40 by the SPP. In vitro–translated pre-atTic40 (lanes 1 and 2), int-atTic40 (lanes 3 and 4), or pretriose phosphate translocator (pre-TPT; lanes 5 and 6) was incubated in the presence (+) or absence (−) of a chloroplast stromal extract containing the SPP.
Figure 2.
The int-atTic40 accumulates in a chloroplast soluble compartment. (A) [35S]pre-atTic40 was imported into isolated pea chloroplasts for the times indicated. After import, the chloroplasts were treated with 50 μg/ml trypsin and reisolated, and the total fraction (T) was separated into membrane (M) or soluble (S) fractions by osmotic lysis. The samples were analyzed by SDS-PAGE and phosphorimaging. Lane 1 contains 20% of [35S]-labeled pre-atTic40 (IVT) added to the original import reaction. (B) Quantitative analysis of the distribution of mature atTic40 (left) or int-atTic40 (right) between the soluble and membrane fractions of chloroplasts. N.D. indicates that the sample was below accurate detection levels.
Figure 3.
Int-atTic40 is an intermediate in the import and membrane targeting of mature atTic40. (A) [35S]pre-atTic40 was incubated with isolated chloroplasts in the presence of 100 μM ATP to promote formation of an early import intermediate. The chloroplasts were isolated and incubated in the presence of 2 mM ATP to promote import of the bound pre-atTic40 for the times indicated (chase). The chloroplasts were separated into membrane and soluble fractions by osmotic lysis and analyzed by SDS-PAGE and phosphorimaging. (B) Quantitative analysis of the total amounts of [35S]-labeled pre-atTic40, int-atTic40, and mature atTic40 in the combined soluble and membrane fractions from A. (C) Samples from the same import experiment in A, but the intact chloroplasts were treated with 100 μg/ml thermolysin before separation into membrane and soluble fractions. (D) Quantitative analysis of the percentage of protease-resistant int-atTic40 or mature atTic40 (from C) relative to the total amount of each species (from A) at each time point in the import experiment. The samples in A and C were generated from the same import experiment and were analyzed on the same SDS-PAGE gel.
Figure 4.
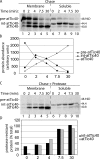
Soluble int-atTic40 is the immediate precursor to membrane-inserted atTic40. (A) [35S]pre-atTic40 was imported into isolated pea chloroplasts for 5 min. The chloroplasts were treated with trypsin, reisolated, and incubated under import conditions for the times indicated. After import, the total chloroplasts (T) were separated into soluble (S) and membrane (M) fractions. Lanes 2–4 contain total, membrane, and soluble fractions from the 5-min import reaction before protease treatment and subsequent incubation. (B) Quantitative analysis of the levels of total int-atTic40 and mature atTic40 at each time point in the postimport incubation of trypsin-treated chloroplasts.
Figure 5.
The intermediate region of the int-atTic40 transit peptide is not required for membrane targeting. (A) [35S]-labeled pre-atTic40 (FL) and deletion mutants spanning the indicated amino acids of pre-atTic40 were imported into isolated chloroplasts for 30 min and subsequently treated in the presence (+) or absence (−) of trypsin. IVT, 20% of the in vitro–translated protein used in each reaction. The graph presents quantitative analysis of the import efficiency of each construct presented as a percentage of the amount of import observed with wild-type pre-atTic40. (B) Analysis of total (T), soluble (S), and membrane (M) fractions of chloroplasts from 30-min import reactions of [35S]-labeled pre-atTic40 and the transit peptide deletion mutants. The graph presents quantitative analysis of the distribution of the total atTic40 species between membrane and soluble fractions for each construct. (C) [35S]pre-atTic40 and [35S]pre-atTic40–Δ58-76 were imported into isolated pea chloroplasts for 5 min, treated with trypsin, reisolated, and incubated under import conditions for the times indicated. The total chloroplasts were separated into soluble and membrane fractions by alkaline extraction. Lanes 2 and 10 contain total (T) chloroplasts from the 5-min import reaction before protease treatment and subsequent incubation. Lanes 1 and 9 contain 20% of the translation product used for each of the import reactions.
Figure 6.
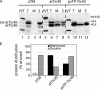
The transmembrane helix of atTic40 is required for membrane targeting. (A) [35S]-labeled pre-atTic40 (atTic40), mature atTic40 fused to the transit peptide of SSU (psTP-Tic40), or a deletion mutant of pre-atTic40 lacking its transmembrane segment (ΔTM) were imported into chloroplasts for 30 min. After import, the chloroplasts were treated with thermolysin, reisolated, and total (T), membrane (M), or soluble (S) fractions were analyzed by SDS-PAGE and phosphorimaging. IVT, 20% of the in vitro–translated protein used in each reaction. (B) Quantitative analysis of distribution of each protein between the membrane and soluble fractions of chloroplasts after import. N.D. indicates that the sample was below accurate detection levels.
Figure 7.
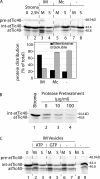
Int-atTic40 can insert into isolated inverted inner membrane vesicles. (A) Isolated IM vesicles are inside out. (i) IM vesicles (20 μg protein) were treated in the presence or absence of trypsin (1 μg trypsin/mg IM protein) or thermolysin (10 μg thermolysin/mg IM protein) on ice for 30 min. The samples were analyzed by SDS-PAGE and immunoblotting with psTic110 or psTic22 antiserum. (ii) IM vesicles were isolated from chloroplasts containing imported [35S]atTic110 and treated in the presence or absence of thermolysin as in panel i for the times indicated. The levels of [35S]atTic110 were detected and quantified using SDS-PAGE and phosphorimaging. (B) [35S]int-atTic40 from a stromal extract binds to IM vesicles. A stromal extract (50 μg protein) containing [35S]int-atTic40 was incubated with isolated IM vesicles (30 μg protein) for the times indicated. The quantitative analysis presents the distribution of int-atTic40 between soluble (S) and membrane (M) fractions at each time point in the insertion reaction. Lane 1 contains a sample of the total chloroplasts (CP) used as the source for the stromal extract. Lane 2 contains a sample of stromal extract equivalent to that added to the IM vesicle insertion reaction. Lanes 9 and 10 present the distribution of int-atTic40 from a reaction that lacked added IM vesicles. (C) IM vesicles from an int-atTic40 insertion reaction similar to that in B were incubated in the absence (−) or presence of alkaline buffer to extract peripheral proteins (pH 11.5) or with thermolysin to test int-atTic40 topology (T-lysin). The samples were separated into membrane and soluble fractions after the treatments.
Figure 8.
The insertion of int-atTic40 into IM vesicles is selective and requires proteinaceous components at the membrane. (A) Int-atTic40 does not bind to canine pancreatic microsomes. A stromal extract containing [35S]int-atTic40 was incubated in the absence (−) or presence of isolated IM vesicles (30 μg protein) or canine pancreatic microsomes (Mc; 30 μg protein) for 2.5 h. After the reaction, the samples were separated into membrane (M) and soluble (S) fractions. The quantitative analysis presents the distribution of combined [35S]-labeled int-atTic40 and atTic40 between the membrane and soluble fractions from each reaction. (B) Int-atTic40 insertion into IM vesicles relies on protease-sensitive membrane components. Isolated IM vesicles were treated with the indicated concentrations of thermolysin before incubation with stromal extract containing [35S]int-atTic40. The fraction of [35S]int-atTic40 that associated with the membranes after alkaline extraction is shown in lanes 2–4. Lane 1 contains a sample of stromal extract equivalent to that added to each reaction. (C) Int-atTic40 targeting to IM vesicles does not require exogenous nucleoside triphosphates. A dialyzed stromal extract containing [35S]int-atTic40 was incubated with IM vesicles in the absence (−) or presence of 2 mM ATP or GTP as indicated. The reactions were separated into membrane and soluble fractions by alkaline extraction and analyzed by SDS-PAGE and phosphorimaging. Lane 1 contains a sample of stromal extract equivalent to that added to each reaction. Lanes 8 and 9 contains stromal extract that was incubated in the absence of IM vesicles and subsequently treated with alkaline buffer.
Figure 9.
atTic40 targeting to IM vesicles does not require stromal components. Stromal extract containing [35S]int-atTic40 or in vitro–translated [35S]int-atTic40, [35S]atTic40, or [35S]ΔTM-atTic40 (reticulocyte lysate) was incubated with isolated IM vesicles for 2.5 h as described in Fig. 7. The total (T) and alkaline-resistant fraction (M) that associates with IM vesicles is shown. IVT, 20% of the in vitro–translation products added to each targeting assay.
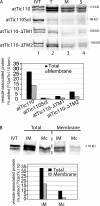
Figure 10.
The inner membrane protein, atTic110, also targets to isolated IM vesicles. (A) In vitro–translated [35S]-labeled atTic110 or atTic110 lacking one (atTic110-ΔTM1 or atTic110-ΔTM2) or both (atTic110Sol) transmembrane segments was incubated with isolated IM vesicles. The vesicles were recovered by differential centrifugation (T) and separated into membrane (M) and soluble (S) fractions by alkaline extraction. The quantitative analysis presents the amount of total or membrane-integrated [35S]-labeled protein that associated with IM vesicles. (B) In vitro–translated [35S]atTic110 was incubated with IM vesicles (30 μg protein) or canine pancreatic microsomal membranes (Mc; 30 μg protein). The vesicles were recovered by differential centrifugation (total) and extracted with alkaline buffer (membrane). The quantitative analysis presents the amount of total or membrane-integrated [35S]-labeled protein that associated with IM vesicles.
Similar articles
- Determinants for stop-transfer and post-import pathways for protein targeting to the chloroplast inner envelope membrane.
Viana AA, Li M, Schnell DJ. Viana AA, et al. J Biol Chem. 2010 Apr 23;285(17):12948-60. doi: 10.1074/jbc.M110.109744. Epub 2010 Mar 1. J Biol Chem. 2010. PMID: 20194502 Free PMC article. - A novel serine/proline-rich domain in combination with a transmembrane domain is required for the insertion of AtTic40 into the inner envelope membrane of chloroplasts.
Tripp J, Inoue K, Keegstra K, Froehlich JE. Tripp J, et al. Plant J. 2007 Dec;52(5):824-38. doi: 10.1111/j.1365-313X.2007.03279.x. Epub 2007 Sep 19. Plant J. 2007. PMID: 17883373 - Tic40 is important for reinsertion of proteins from the chloroplast stroma into the inner membrane.
Chiu CC, Li HM. Chiu CC, et al. Plant J. 2008 Dec;56(5):793-801. doi: 10.1111/j.1365-313X.2008.03638.x. Epub 2008 Jul 23. Plant J. 2008. PMID: 18657235 Free PMC article. - Targeting and biogenesis of transporters and channels in chloroplast envelope membranes: Unsolved questions.
Oh YJ, Hwang I. Oh YJ, et al. Cell Calcium. 2015 Jul;58(1):122-30. doi: 10.1016/j.ceca.2014.10.012. Epub 2014 Oct 31. Cell Calcium. 2015. PMID: 25465895 Review. - Mechanisms of protein import and routing in chloroplasts.
Jarvis P, Robinson C. Jarvis P, et al. Curr Biol. 2004 Dec 29;14(24):R1064-77. doi: 10.1016/j.cub.2004.11.049. Curr Biol. 2004. PMID: 15620643 Review.
Cited by
- The role of GTP binding and hydrolysis at the atToc159 preprotein receptor during protein import into chloroplasts.
Wang F, Agne B, Kessler F, Schnell DJ. Wang F, et al. J Cell Biol. 2008 Oct 6;183(1):87-99. doi: 10.1083/jcb.200803034. Epub 2008 Sep 29. J Cell Biol. 2008. PMID: 18824565 Free PMC article. - Evolutionary, molecular and genetic analyses of Tic22 homologues in Arabidopsis thaliana chloroplasts.
Kasmati AR, Töpel M, Khan NZ, Patel R, Ling Q, Karim S, Aronsson H, Jarvis P. Kasmati AR, et al. PLoS One. 2013 May 13;8(5):e63863. doi: 10.1371/journal.pone.0063863. Print 2013. PLoS One. 2013. PMID: 23675512 Free PMC article. - Evolution of protein transport to the chloroplast envelope membranes.
Day PM, Theg SM. Day PM, et al. Photosynth Res. 2018 Dec;138(3):315-326. doi: 10.1007/s11120-018-0540-x. Epub 2018 Oct 5. Photosynth Res. 2018. PMID: 30291507 Review. - Chloroplast Hsp93 Directly Binds to Transit Peptides at an Early Stage of the Preprotein Import Process.
Huang PK, Chan PT, Su PH, Chen LJ, Li HM. Huang PK, et al. Plant Physiol. 2016 Feb;170(2):857-66. doi: 10.1104/pp.15.01830. Epub 2015 Dec 16. Plant Physiol. 2016. PMID: 26676256 Free PMC article. - Differential age-dependent import regulation by signal peptides.
Teng YS, Chan PT, Li HM. Teng YS, et al. PLoS Biol. 2012;10(10):e1001416. doi: 10.1371/journal.pbio.1001416. Epub 2012 Oct 30. PLoS Biol. 2012. PMID: 23118617 Free PMC article.
References
- Baumann, F., W. Neupert, and J.M. Herrmann. 2002. Insertion of bitopic membrane proteins into the inner membrane of mitochondria involves an export step from the matrix. J. Biol. Chem. 277:21405–21413. - PubMed
- Bedard, J., and P. Jarvis. 2005. Recognition and envelope translocation of chloroplast preproteins. J. Exp. Bot. 56:2287–2320. - PubMed
- Bolter, B., A. Nada, H. Fulgosi, and J. Soll. 2006. A chloroplastic inner envelope membrane protease is essential for plant development. FEBS Lett. 580:789–794. - PubMed
- Brink, S., K. Fischer, R.-B. Klosgen, and U.-I. Flugge. 1995. Sorting of nuclear-encoded chloroplast membrane proteins to the envelope and the thylakoid membrane. J. Biol. Chem. 270:20808–20815. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases