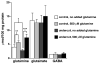Modulation of epileptiform activity by glutamine and system A transport in a model of post-traumatic epilepsy - PubMed (original) (raw)
Modulation of epileptiform activity by glutamine and system A transport in a model of post-traumatic epilepsy
Hiroaki Tani et al. Neurobiol Dis. 2007 Feb.
Abstract
Epileptic activity arises from an imbalance in excitatory and inhibitory synaptic transmission. To determine if alterations in the metabolism of glutamate, the primary excitatory neurotransmitter, might contribute to epilepsy we directly and indirectly modified levels of glutamine, an immediate precursor of synaptically released glutamate, in the rat neocortical undercut model of hyperexcitability and epilepsy. We show that slices from injured cortex take up glutamine more readily than control slices, and an increased expression of the system A transporters SNAT1 and SNAT2 likely underlies this difference. We also examined the effect of exogenous glutamine on evoked and spontaneous activity and found that addition of physiological concentrations of glutamine to perfusate of slices isolated from injured cortex increased the incidence and decreased the refractory period of epileptiform potentials. By contrast, exogenous glutamine increased the amplitude of evoked potentials in normal cortex, but did not induce epileptiform potentials. Addition of physiological concentrations of glutamine to perfusate of slices isolated from injured cortex greatly increased abnormal spontaneous activity in the form of events resembling spreading depression, again while having no effect on slices from normal cortex. Interestingly, similar spreading depression like events were noted in control slices at supraphysiological levels of glutamine. In the undercut cortex addition of methylaminoisobutyric acid (MeAIB), an inhibitor of the system A glutamine transporters attenuated all physiological effects of added glutamine suggesting that uptake through these transporters is required for the effect of glutamine. Our findings support a role for glutamine transport through SNAT1 and/or SNAT2 in the maintenance of abnormal activity in this in vitro model of epileptogenesis and suggest that system A transport and glutamine metabolism are potential targets for pharmacological intervention in seizures and epilepsy.
Figures
Figure 1. Exogenous glutamine leads to an glutamine concentration in control and undercut slices
Amino acid levels were measured in ethanol extracts from control (n=6) and undercut slices (n=6) incubated in oxygenated interface chambers for 2 hours in ACSF with or without 0.5 mM glutamine. Results demonstrate that in the absence of added glutamine, tissue levels of the amino acid are significantly lower (p<0.05, *) in undercut samples than in controls. There are also statistically significant increases (p<0.03** for control and p<0.0003*** for undercut) in glutamine levels in both control and undercut tissue in response to exogenous glutamine with a 50% increase in glutamine content in control slices and a 160% increase in undercut slices. Differences in glutamine concentrations in the control versus undercut slices treated with glutamine are statistically significant (p<0.05), but glutamate and GABA levels are not significantly altered in either undercut or control slices in response to glutamine.
Figure 2. System A and System N transporters are upregulated in undercut cortex
Digoxigenin labeled probes for mRNAs of the system A transporters SNAT1 (A) and SNAT2 (B) were used to assess expression of the transporter genes by in situ hybridization. Examination of cortex adjacent to the undercut lesion (right panels) demonstrates an increased signal in deep cortical layers (black arrows top panel, white arrows bottom panel) when compared to the contralateral control cortex (left panels) for both transporter mRNAs. The scale bars indicate 500μm for the low power images and 100μm for high power images.
Figure 3. SNAT1 and SNAT2 protein are increased in the undercut cortex
Immunostaining with SNAT1 and SNAT2 specific antibodies (A) demonstrates a marked increase in SNAT1 (upper panels) and SNAT2 (lower panels) immunoreactivity in layer V neurons of undercut cortex (right panels) compared to contralateral control cortex in the same sections (left panels). With the same antibodies western blotting of protein extracts from 3 control (left) and 3 undercut cortex samples each from individual animals demonstrate a more marked increase in SNAT2 (lower panel) compared to SNAT1 (upper panel). For immunofluorescence images scale bar indicates 20μm.
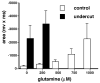
Figure 4. Glutamine increases the size of primary evoked potentials in control and undercut cortex
Measurements of mean primary evoked response area (mV × ms) from control and undercut slices demonstrate larger potentials in the undercut slices in the absence of glutamine. Addition of glutamine leads to increases for both with the size of the potentials increasing with glutamine concentrations of up to 1mM. At concentrations of 500μM and above glutamine induced spreading depression like events in undercut cortex and precluded an appropriate analysis. Data were collected from four independent slices for each group. The primary evoked response was defined as that occurring within 50 μs of the stimulus.
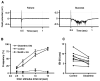
Figure 5. Glutamine and MeAIB have opposing effects on the likelihood of polyphasic potentials in undercut cortex
Sample evoked potentials from undercut cortex field recordings (A) demonstrate a simple monophasic response either without (failure, left) or with (success, right) a late polyphasic response. B) Incidence of polyphasic events in undercut slices in response to varying interstimulus intervals (ISIs). Slices were exposed to either 250μM glutamine or 250μM glutamine with 10mM MeAIB in the ACSF (n=5 from 4 animals). Addition of glutamine increased the incidence of polyphasic events causing a leftward shift in the curve indicating a decrease the refractory period. By contrast, addition of MeAIB almost completely blocked polyphasic activity. C) The interval evoking 50% successes (ISI-50) was consistently shortened by 250 μM glutamine, as shown in this paired analysis. The ISI-50 decreased from 8.2 seconds to 5.9 seconds. Student's two-tailed t-test p<0.01, n=8 slices from 4 animals.
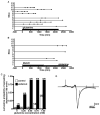
Figure 6. Glutamine induces spreading depression like events, which are attenuated by MeAIB
A) Analysis of undercut slices demonstrates frequent large negative field potentials (e.g. panel D) after the addition of 250μM glutamine in 9 of 13 undercut slices from 8 animals. B) This effect is attenuated by the addition of MeAIB (10mM) with no events in any of 13 slices during 20 minute recording periods. After washout of MeAIB in the continued presence of 250 μM glutamine, 4 slices exhibit at least one large depolarization event. Each slice was recorded for a total of 45 minutes after initial exposure to glutamine. Cumulative event analysis demonstrates spreading depression like events in control and undercut cortex, with events appearing more frequently as glutamine concentration is increased. Field recordings were monitored for 10 min at each glutamine concentration. D). Sample tracing of a spreading depression event depicts typical negative potential lasting ~30 sec. Brief transient events are stimulus artifacts associated with evoked synaptic potentials.
Similar articles
- Inhibition of glutamine transport depletes glutamate and GABA neurotransmitter pools: further evidence for metabolic compartmentation.
Rae C, Hare N, Bubb WA, McEwan SR, Bröer A, McQuillan JA, Balcar VJ, Conigrave AD, Bröer S. Rae C, et al. J Neurochem. 2003 Apr;85(2):503-14. doi: 10.1046/j.1471-4159.2003.01713.x. J Neurochem. 2003. PMID: 12675927 - The glutamine commute: lost in the tube?
Conti F, Melone M. Conti F, et al. Neurochem Int. 2006 May-Jun;48(6-7):459-64. doi: 10.1016/j.neuint.2005.11.016. Epub 2006 Mar 3. Neurochem Int. 2006. PMID: 16517023 Review. - SNAT2 amino acid transporter is regulated by amino acids of the SLC6 gamma-aminobutyric acid transporter subfamily in neocortical neurons and may play no role in delivering glutamine for glutamatergic transmission.
Grewal S, Defamie N, Zhang X, De Gois S, Shawki A, Mackenzie B, Chen C, Varoqui H, Erickson JD. Grewal S, et al. J Biol Chem. 2009 Apr 24;284(17):11224-36. doi: 10.1074/jbc.M806470200. Epub 2009 Feb 24. J Biol Chem. 2009. PMID: 19240036 Free PMC article. - System A transporter SAT2 mediates replenishment of dendritic glutamate pools controlling retrograde signaling by glutamate.
Jenstad M, Quazi AZ, Zilberter M, Haglerød C, Berghuis P, Saddique N, Goiny M, Buntup D, Davanger S, S Haug FM, Barnes CA, McNaughton BL, Ottersen OP, Storm-Mathisen J, Harkany T, Chaudhry FA. Jenstad M, et al. Cereb Cortex. 2009 May;19(5):1092-106. doi: 10.1093/cercor/bhn151. Epub 2008 Oct 1. Cereb Cortex. 2009. PMID: 18832333 - Epilepsy following cortical injury: cellular and molecular mechanisms as targets for potential prophylaxis.
Prince DA, Parada I, Scalise K, Graber K, Jin X, Shen F. Prince DA, et al. Epilepsia. 2009 Feb;50 Suppl 2(Suppl 2):30-40. doi: 10.1111/j.1528-1167.2008.02008.x. Epilepsia. 2009. PMID: 19187292 Free PMC article. Review.
Cited by
- Disinhibition reduces extracellular glutamine and elevates extracellular glutamate in rat hippocampus in vivo.
Kanamori K. Kanamori K. Epilepsy Res. 2015 Aug;114:32-46. doi: 10.1016/j.eplepsyres.2015.03.009. Epub 2015 Mar 23. Epilepsy Res. 2015. PMID: 26088883 Free PMC article. - Exploring the pathogenesis of canine epilepsy using a systems genetics method and implications for anti-epilepsy drug discovery.
Cui ZJ, Liu YM, Zhu Q, Xia J, Zhang HY. Cui ZJ, et al. Oncotarget. 2017 Dec 27;9(17):13181-13192. doi: 10.18632/oncotarget.23719. eCollection 2018 Mar 2. Oncotarget. 2017. PMID: 29568349 Free PMC article. - Mechanisms of Excessive Extracellular Glutamate Accumulation in Temporal Lobe Epilepsy.
Albrecht J, Zielińska M. Albrecht J, et al. Neurochem Res. 2017 Jun;42(6):1724-1734. doi: 10.1007/s11064-016-2105-8. Epub 2016 Nov 21. Neurochem Res. 2017. PMID: 27873132 Review. - Riluzole and novel naphthalenyl substituted aminothiazole derivatives prevent acute neural excitotoxic injury in a rat model of temporal lobe epilepsy.
Kyllo T, Singh V, Shim H, Latika S, Nguyen HM, Chen YJ, Terry E, Wulff H, Erickson JD. Kyllo T, et al. Neuropharmacology. 2023 Feb 15;224:109349. doi: 10.1016/j.neuropharm.2022.109349. Epub 2022 Nov 24. Neuropharmacology. 2023. PMID: 36436594 Free PMC article. - Maintenance of thalamic epileptiform activity depends on the astrocytic glutamate-glutamine cycle.
Bryant AS, Li B, Beenhakker MP, Huguenard JR. Bryant AS, et al. J Neurophysiol. 2009 Nov;102(5):2880-8. doi: 10.1152/jn.00476.2009. Epub 2009 Sep 9. J Neurophysiol. 2009. PMID: 19741104 Free PMC article.
References
- Bacci A, Sancini G, Verderio C, Armano S, Pravettoni E, Fesce R, Franceschetti S, Matteoli M. Block of glutamate-glutamine cycle between astrocytes and neurons inhibits epileptiform activity in hippocampus. J Neurophysiol. 2002;88:2302–2310. - PubMed
- Broer S, Brookes N. Transfer of glutamine between astrocytes and neurons. J Neurochem. 2001;77:705–719. - PubMed
- Chaudhry FA, Lehre KP, vanLookeren Campagne M, Otterson OP, Danbolt NC, Storm-Mathisen J. Glutamate transporters in glial plasma membranes: highly differentiated localizations revealed by quantitative ultrastructural immunocytochemistry. Neuron. 1995;15:711–720. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS012151/NS/NINDS NIH HHS/United States
- NS045634/NS/NINDS NIH HHS/United States
- P01 NS012151/NS/NINDS NIH HHS/United States
- P50 NS012151/NS/NINDS NIH HHS/United States
- K02 NS045634/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources