Mitochondria contribute to LPS-induced MAPK activation via uncoupling protein UCP2 in macrophages - PubMed (original) (raw)
Mitochondria contribute to LPS-induced MAPK activation via uncoupling protein UCP2 in macrophages
Yalin Emre et al. Biochem J. 2007.
Abstract
The mitochondrion is a major organelle contributing to energy metabolism but also a main site of ROS (reactive oxygen species) production. LPS (lipopolysaccharide)-induced ROS signalling is a critical event in macrophage activation. In the present paper we report that part of LPS-mediated ROS signalling comes from mitochondria inside a signal amplification loop that enhances MAPK (mitogen-activated protein kinase) activation. More precisely, we have identified the inner mitochondrial membrane UCP2 (uncoupling protein 2) as a physiological brake on ROS signalling. Stimulation of murine bone marrow-derived macrophages by LPS quickly down-regulated UCP2 through the JNK (c-Jun N-terminal kinase) and p38 pathways. UCP2 down-regulation was shown to be necessary to increase mitochondrial ROS production in order to potentiate MAPK activation. Consistent with this, UCP2-deficient macrophages exhibit an enhanced inflammatory state characterized by increased nitric oxide production and elevated migration ability. Additionally, we found that the absence of UCP2 renders macrophages more resistant to nitric oxide-induced apoptosis.
Figures
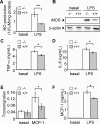
Figure 1. Ucp2−/− macrophages exhibit an increased inflammatory response
(A–D) Macrophages were stimulated with LPS (1 μg/ml) or left unstimulated for 24 h. NO production (A) was determined (_n_=4) and iNOS protein (B) was quantified by Western blot analysis. The amount of β-actin served as a loading control. Western blots represent the results of one experiment representative of four independent experiments. TNF-α (C) and IL-6 (D) secretions of macrophages were measured by ELISA (_n_=3). (E) Transmigration of macrophages (_A_550 nm) through an endothelial cell monolayer in the presence or absence of MCP-1 was determined as described in the Experimental procedures section (_n_=3). (F) MCP-1 release of macrophages stimulated with LPS (1 μg/ml) or unstimulated for 24 h was measured by ELISA (_n_=3). *P<0.05, **P<0.01.
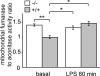
Figure 2. LPS-induced mitochondrial ROS production is controlled by UCP2
Macrophages were stimulated with LPS (1 μg/ml) or unstimulated for 60 min. Mitochondrial ROS production was measured as the ratio of mitochondrial fumarase/aconitase activities as described in the Experimental procedures section (_n_=4). *P<0.05, **P<0.01.
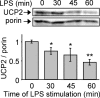
Figure 3. Macrophage activation by LPS leads to early down-regulation of UCP2
Mitochondrial fractions were prepared prior to immunoblotting. Immunoblot analysis of UCP2 protein after LPS treatment (1 μg/ml). The amount of porin served as a loading control. Western blots represent the results of one experiment representative of four independent experiments. *P<0.05, **P<0.01.
Figure 4. The NF-κB signalling pathway is not modified in Ucp2−/− macrophages
Macrophages were stimulated with LPS (1 μg/ml) for the indicated time periods. (A) IκB degradation was analysed by Western blot. The amount of β-actin served as a loading control. Western blots represent the results of one experiment representative of three independent experiments. (B) NF-κB DNA binding activity in nuclei of macrophages stimulated with LPS (1 μg/ml) or unstimulated was analysed as described in the Experimental section (_n_=2). AU, arbitrary units.
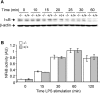
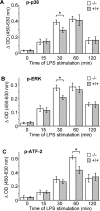
Figure 5. LPS-induced p38, ERK and ATF-2 phosphorylation is higher in Ucp2−/− macrophages
Macrophages were stimulated with LPS (1 μg/ml) for the indicated time periods. (A) Phosphorylation of p38 (p-p38), (B) phosphorylation of ERK (p-ERK), and (C) phosphorylation of ATF-2 (p-ATF-2), was determined by an ELISA-based method as described in the Experimental section (_n_=3). *P<0.05.
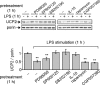
Figure 6. UCP2 down-regulation is mediated by the p38 and JNK signalling pathways
Immunoblot analysis of UCP2 protein after 1 h of LPS (1 μg/ml) stimulation of macrophages pre-incubated with vehicle, IL-10 (100 ng/ml) or inhibitors of ERK (PD98059; 50 μM), JNK (SP600125; 25 μM), p38 (SB202190; 10 μM), mTOR (rapamycin; 1 μM) or Mnk-1 (CGP057380; 10 μM), (_n_=3).
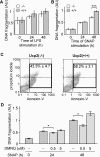
Figure 7. Ucp2−/− macrophages are more resistant to NO-induced apoptosis
(A) DNA fragmentation in macrophages stimulated with LPS (1 μg/ml) was analysed by ELISA as described in the Experimental section (_n_=3). (B and C) Macrophages were treated with SNAP (50 μM), an NO donor, for the indicated times. (B) DNA fragmentation was analysed by ELISA (_n_=5). (C) Annexin-V and propidium iodide staining was analysed by flow cytometry 48 h after SNAP treatment (_n_=5). (D) Apoptosis was induced by treating Ucp2+/+ macrophages with SNAP (50 μM) in the presence or absence of different concentrations of DMNQ, a ROS donor. DNA fragmentation was analysed by ELISA (_n_≥3). *P<0.05, ***P<0.005.
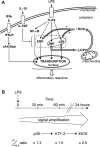
Figure 8. Model for the role of UCP2 in LPS-induced mitochondrial ROS signalling
(A) Recognition of LPS by TLR-4 activates JNK, p38 and ERK signalling pathways. Meanwhile, a ROS signalling pathway is set up. UCP2 is quickly down-regulated in response to LPS via the JNK and p38 pathways to increase mitochondrial ROS production. The mitochondrial part of this signal is regulated by UCP2, a physiological brake that has to be switched off in order to enhance mitochondrial ROS production. This mitochondrial ROS generation, as a feedback signal, stimulates the ERK and p38 pathways. In this way, mitochondria contribute to the macrophage inflammatory response. (B) Diagram showing the amplified signalling cascade responsible for the enhanced inflammatory state of Ucp2−/− macrophages.
Similar articles
- Taxol-induced mitochondrial stress in melanoma cells is mediated by activation of c-Jun N-terminal kinase (JNK) and p38 pathways via uncoupling protein 2.
Selimovic D, Hassan M, Haikel Y, Hengge UR. Selimovic D, et al. Cell Signal. 2008 Feb;20(2):311-22. doi: 10.1016/j.cellsig.2007.10.015. Epub 2007 Oct 17. Cell Signal. 2008. PMID: 18068334 - The mitochondrial uncoupling protein-2 is a master regulator of both M1 and M2 microglial responses.
De Simone R, Ajmone-Cat MA, Pandolfi M, Bernardo A, De Nuccio C, Minghetti L, Visentin S. De Simone R, et al. J Neurochem. 2015 Oct;135(1):147-56. doi: 10.1111/jnc.13244. Epub 2015 Aug 3. J Neurochem. 2015. PMID: 26173855 - Uncoupling protein 2 negatively regulates mitochondrial reactive oxygen species generation and induces phosphatase-mediated anti-inflammatory response in experimental visceral leishmaniasis.
Basu Ball W, Kar S, Mukherjee M, Chande AG, Mukhopadhyaya R, Das PK. Basu Ball W, et al. J Immunol. 2011 Aug 1;187(3):1322-32. doi: 10.4049/jimmunol.1004237. Epub 2011 Jun 24. J Immunol. 2011. PMID: 21705615 - Uncoupling protein UCP2: when mitochondrial activity meets immunity.
Emre Y, Nübel T. Emre Y, et al. FEBS Lett. 2010 Apr 16;584(8):1437-42. doi: 10.1016/j.febslet.2010.03.014. Epub 2010 Mar 15. FEBS Lett. 2010. PMID: 20227410 Review. - Mitochondrial UCP2 in the central regulation of metabolism.
Toda C, Diano S. Toda C, et al. Best Pract Res Clin Endocrinol Metab. 2014 Oct;28(5):757-64. doi: 10.1016/j.beem.2014.02.006. Epub 2014 Mar 7. Best Pract Res Clin Endocrinol Metab. 2014. PMID: 25256770 Review.
Cited by
- Readily accessible fluorescent probes for sensitive biological imaging of hydrogen peroxide.
Daniel KB, Agrawal A, Manchester M, Cohen SM. Daniel KB, et al. Chembiochem. 2013 Mar 18;14(5):593-8. doi: 10.1002/cbic.201200724. Epub 2013 Feb 21. Chembiochem. 2013. PMID: 23436442 Free PMC article. - MEK6 Overexpression Exacerbates Fat Accumulation and Inflammatory Cytokines in High-Fat Diet-Induced Obesity.
Lee S, Lee M. Lee S, et al. Int J Mol Sci. 2021 Dec 17;22(24):13559. doi: 10.3390/ijms222413559. Int J Mol Sci. 2021. PMID: 34948353 Free PMC article. - Kupffer cells in non-alcoholic fatty liver disease: the emerging view.
Baffy G. Baffy G. J Hepatol. 2009 Jul;51(1):212-23. doi: 10.1016/j.jhep.2009.03.008. Epub 2009 Mar 31. J Hepatol. 2009. PMID: 19447517 Free PMC article. Review. - Deletion of UCP2 in iNOS deficient mice reduces the severity of the disease during experimental autoimmune encephalomyelitis.
Aheng C, Ly N, Kelly M, Ibrahim S, Ricquier D, Alves-Guerra MC, Miroux B. Aheng C, et al. PLoS One. 2011;6(8):e22841. doi: 10.1371/journal.pone.0022841. Epub 2011 Aug 8. PLoS One. 2011. PMID: 21857957 Free PMC article. - The emerging role for metabolism in fueling neutrophilic inflammation.
Morrison T, Watts ER, Sadiku P, Walmsley SR. Morrison T, et al. Immunol Rev. 2023 Mar;314(1):427-441. doi: 10.1111/imr.13157. Epub 2022 Nov 3. Immunol Rev. 2023. PMID: 36326284 Free PMC article. Review.
References
- Gordon S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003;3:23–35. - PubMed
- Takeda K., Kaisho T., Akira S. Toll-like receptors. Annu. Rev. Immunol. 2003;21:335–376. - PubMed
- Medzhitov R. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 2001;1:135–145. - PubMed
- Poltorak A., He X., Smirnova I., Liu M. Y., Van Huffel C., Du X., Birdwell D., Alejos E., Silva M., et al. Limits of a deletion spanning Tlr4 in C57BL/10ScCr mice. Science. 1998;282:2085–2088. - PubMed
- Guha M., Mackman N. LPS induction of gene expression in human monocytes. Cell. Signalling. 2001;13:85–94. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous