Secreted PCSK9 decreases the number of LDL receptors in hepatocytes and in livers of parabiotic mice - PubMed (original) (raw)
Secreted PCSK9 decreases the number of LDL receptors in hepatocytes and in livers of parabiotic mice
Thomas A Lagace et al. J Clin Invest. 2006 Nov.
Abstract
Proprotein convertase subtilisin/kexin type 9 (PCSK9) is a member of the proteinase K subfamily of subtilases that reduces the number of LDL receptors (LDLRs) in liver through an undefined posttranscriptional mechanism. We show that purified PCSK9 added to the medium of HepG2 cells reduces the number of cell-surface LDLRs in a dose- and time-dependent manner. This activity was approximately 10-fold greater for a gain-of-function mutant, PCSK9(D374Y), that causes hypercholesterolemia. Binding and uptake of PCSK9 were largely dependent on the presence of LDLRs. Coimmunoprecipitation and ligand blotting studies indicated that PCSK9 and LDLR directly associate; both proteins colocalized to late endocytic compartments. Purified PCSK9 had no effect on cell-surface LDLRs in hepatocytes lacking autosomal recessive hypercholesterolemia (ARH), an adaptor protein required for endocytosis of the receptor. Transgenic mice overexpressing human PCSK9 in liver secreted large amounts of the protein into plasma, which increased plasma LDL cholesterol concentrations to levels similar to those of LDLR-knockout mice. To determine whether PCSK9 was active in plasma, transgenic PCSK9 mice were parabiosed with wild-type littermates. After parabiosis, secreted PCSK9 was transferred to the circulation of wild-type mice and reduced the number of hepatic LDLRs to nearly undetectable levels. We conclude that secreted PCSK9 associates with the LDLR and reduces hepatic LDLR protein levels.
Figures
Figure 1. Pulse-chase analysis of endogenous PCSK9 secretion from HepG2 cells.
Cells were cultured in sterol-depleting medium C for 18 hours prior to labeling for 30 minutes with [35S]methionine/cysteine. After washing, cells were incubated in chase medium containing unlabeled methionine and cysteine for the indicated times. Cells were lysed, and PCSK9 was immunoprecipitated from the medium and cell lysates as described in Methods. Samples were subjected to 8% SDS-PAGE, and the gel was treated with Amplify fluorogenic reagent prior to drying and exposure to film. P and C denote the proprotein and cleaved forms of PCSK9, respectively. Similar results were obtained in 4 independent experiments.
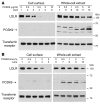
Figure 2. Reduction of endogenous LDLRs in HepG2 cells following the addition of recombinant purified PCSK9 to the culture medium.
(A) Dose response of exogenous PCSK9-mediated LDLR degradation in HepG2 cells. Cells were cultured for 18 hours in medium C prior to treatment for 4 hours with the indicated amounts of purified human PCSK9. (B) Time course of exogenous PCSK9-mediated LDLR degradation. HepG2 cells cultured as described above were treated with 5 μg/ml of purified PCSK9 for the indicated times. Total lysates were prepared following cell-surface biotinylation as described in Methods. Proteins were subjected to SDS-PAGE for immunoblot analysis of the LDLR using IgG-HL1 and anti–FLAG M2 monoclonal antibody to detect purified PCSK9. The transferrin receptor protein was detected as described in Methods and used as a control for loading and nonspecific protein degradation. Similar results were obtained in 3 independent experiments.
Figure 3. Increased cell association and LDLR degradation by addition of purified mutant PCSK9(D374Y) to the medium of HepG2 cells.
Cells were cultured for 18 hours in medium C and then incubated for 4 hours with the indicated amounts of purified human PCSK9 or PCSK9(D374Y). Immunoblot analysis of LDLR, FLAG-tagged PCSK9, and transferrin receptor was carried out as described in the legend to Figure 2. The asterisk indicates a nonspecific band. Similar results were obtained in 3 independent experiments.
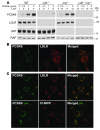
Figure 4. LDLR-dependent endocytosis of PCSK9 in MEFs.
(A) Immunoblot analysis of PCSK9 association with MEFs. Immortalized MEFs derived from wild-type, Ldlr–/–, Lrp–/–, and _Ldlr–/–Lrp–/–_mice were cultured for 18 hours in medium F prior to treatment with purified human PCSK9 for 4 hours. Cell lysates (30 μg) were subjected to SDS-PAGE and immunoblot analysis using IgG-15A6 to detect PCSK9 or a polyclonal antiserum (Ab 3143) that recognizes the LDLR, as described in Methods. Immunoblots of receptor-associated protein (RAP) were used as a loading control. (B) Indirect immunofluorescence localization of PCSK9 and the LDLR in wild-type MEFs. MEFs were incubated for 4 hours with 5 μg/ml purified human PCSK9 and processed for double immunofluorescence of PCSK9 (green) and the LDLR (red) as described in Methods. (C) Indirect immunofluorescence localization of PCSK9, LDLR, and the late-endosomal marker CI-MPR in MEFs cultured in the presence of chloroquine. Wild-type MEFs were incubated for 4 hours with 5 μg/ml purified human PCSK9 in the presence of 0.1 mM chloroquine and processed for double immunofluorescence of PCSK9 (green) and the LDLR (red) or CI-MPR (red) as described in Methods. The merged image shows areas of colocalization of PCSK9 with the LDLR and CI-MPR. Magnification, ×630. Similar results were obtained in 3 independent experiments.
Figure 5. Association of PCSK9 and PCSK9(D374Y) with the LDLR.
(A) Coimmunoprecipitation of the LDLR and exogenously added wild-type PCSK9 or PCSK9(D374Y) protein. HepG2 cells were cultured for 18 hours in medium C prior to treatment for 1 hour in the presence of 0.1 mM chloroquine with 20 μg/ml or 2 μg/ml of purified human PCSK9 or PCSK9(D374Y), respectively. Cells were harvested and lysed and proteins immunoprecipitated with the indicated antibodies. Pellets of the immunoprecipitation were subjected to SDS-PAGE and immunoblot analysis. Polyclonal antiserum (Ab 3143) was used to detect LDLR, and IgG-15A6 was used to detect PCSK9. (B) Reduced (R) or nonreduced (NR) LDLR extracellular domain protein (2 μg) was resolved by SDS-PAGE. LDLR protein was detected with Coomassie brilliant blue R-250 stain. (C) Binding of PCSK9 to LDLR on ligand blots. LDLR protein (2 μg) was transferred to nitrocellulose and incubated with 5 μg/ml of purified PCSK9 or no addition (NA). Bound PCSK9 was detected by immunoblot analysis as described in Methods. (D) The indicated amounts of purified LDLR were subjected to nonreduced SDS-PAGE, transferred to nitrocellulose, and incubated with 5 μg/ml of purified wild-type PCSK9, PCSK9(D374Y), or buffer control (no addition). Bound PCSK9 was detected by immunoblot analysis as described in Methods. pep., FLAG octapeptide.
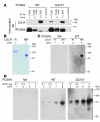
Figure 6. PCSK9-mediated degradation of the LDLR is dependent on ARH.
Immunofluorescence (A) and immunoblot (B) analyses of the PCSK9-mediated changes of LDLR protein in mouse primary hepatocytes derived from LDLRh/hArh+/+(Arh+/+) or LDLRh/hArh–/– (Arh–/–) mice. (A) Primary hepatocytes were incubated with no PCSK9 (–PCSK9) or with 5 μg/ml purified PCSK9 (+PCSK9) for 4 hours and processed for indirect immunofluorescence of LDLR using IgG-C7 and Alexa Fluor 563–conjugated secondary antibody, as described in Methods. Images were taken using a 63 × 1.3 objective using a confocal microscope (Leica TCS SP). Similar results were obtained in 2 independent experiments. (B) Immunoblot analysis of LDLR, FLAG-tagged PCSK9, transferrin receptor, and actin proteins in primary hepatocytes from mice of the indicated genotype incubated with varying amounts of PCSK9.
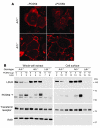
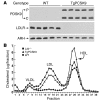
Figure 7. Decreased amounts of hepatic LDLR protein and increased levels of plasma LDL cholesterol in transgenic PCSK9 mice.
(A) Whole-cell lysates (30 μg) from livers of 5 wild-type and 5 TgPCSK9 mice were subjected to SDS-PAGE and immunoblot analyses of human PCSK9, mouse LDLR, and mouse ARH. (B) Plasma from the mice of each genotype described in A and from 5 age-matched male Ldlr–/– mice was pooled and subjected to gel filtration by fast performance liquid chromatography. The concentration of total cholesterol in each fraction was measured as described in Methods.
Figure 8. Amounts of LDLR protein in livers of parabiosed mice.
Three combinations of mice were parabiosed for study: wild-type:wild-type (A); Ldlr–/–:wild-type (B); and TgPCSK9:wild-type (C). Prior to parabiosis, a liver sample (<100 mg) via a partial hepatectomy and approximately 50 μl of blood via the tail vein were obtained from each mouse. After a 7-day recovery period, 12- to 20-week-old female mice of the indicated genotype were parabiosed as described in Methods. After 2 weeks of parabiosis, the animals were killed, and blood and liver samples were collected for analysis. Liver membrane protein (40 μg) obtained from each mouse before (–) and after (+) parabiosis was subjected to SDS-PAGE for immunoblot analyses of LDLR and RAP as a loading control (9).
Similar articles
- Secreted PCSK9 downregulates low density lipoprotein receptor through receptor-mediated endocytosis.
Qian YW, Schmidt RJ, Zhang Y, Chu S, Lin A, Wang H, Wang X, Beyer TP, Bensch WR, Li W, Ehsani ME, Lu D, Konrad RJ, Eacho PI, Moller DE, Karathanasis SK, Cao G. Qian YW, et al. J Lipid Res. 2007 Jul;48(7):1488-98. doi: 10.1194/jlr.M700071-JLR200. Epub 2007 Apr 20. J Lipid Res. 2007. PMID: 17449864 - Secreted proprotein convertase subtilisin/kexin type 9 reduces both hepatic and extrahepatic low-density lipoprotein receptors in vivo.
Schmidt RJ, Beyer TP, Bensch WR, Qian YW, Lin A, Kowala M, Alborn WE, Konrad RJ, Cao G. Schmidt RJ, et al. Biochem Biophys Res Commun. 2008 Jun 13;370(4):634-40. doi: 10.1016/j.bbrc.2008.04.004. Epub 2008 Apr 10. Biochem Biophys Res Commun. 2008. PMID: 18406350 - Decreased plasma cholesterol and hypersensitivity to statins in mice lacking Pcsk9.
Rashid S, Curtis DE, Garuti R, Anderson NN, Bashmakov Y, Ho YK, Hammer RE, Moon YA, Horton JD. Rashid S, et al. Proc Natl Acad Sci U S A. 2005 Apr 12;102(15):5374-9. doi: 10.1073/pnas.0501652102. Epub 2005 Apr 1. Proc Natl Acad Sci U S A. 2005. PMID: 15805190 Free PMC article. - Targeting the proprotein convertase subtilisin/kexin type 9 for the treatment of dyslipidemia and atherosclerosis.
Urban D, Pöss J, Böhm M, Laufs U. Urban D, et al. J Am Coll Cardiol. 2013 Oct 15;62(16):1401-8. doi: 10.1016/j.jacc.2013.07.056. Epub 2013 Aug 21. J Am Coll Cardiol. 2013. PMID: 23973703 Review. - PCSK9 and LDLR degradation: regulatory mechanisms in circulation and in cells.
Lagace TA. Lagace TA. Curr Opin Lipidol. 2014 Oct;25(5):387-93. doi: 10.1097/MOL.0000000000000114. Curr Opin Lipidol. 2014. PMID: 25110901 Free PMC article. Review.
Cited by
- Regulation of epithelial sodium channel trafficking by proprotein convertase subtilisin/kexin type 9 (PCSK9).
Sharotri V, Collier DM, Olson DR, Zhou R, Snyder PM. Sharotri V, et al. J Biol Chem. 2012 Jun 1;287(23):19266-74. doi: 10.1074/jbc.M112.363382. Epub 2012 Apr 9. J Biol Chem. 2012. PMID: 22493497 Free PMC article. - Utilizing HaloTag Technology to Track the Fate of PCSK9 from Intracellular vs. Extracellular Sources.
Ai X, Fischer P, Palyha OC, Wisniewski D, Hubbard B, Akinsanya K, Strack AM, Ehrhardt AG. Ai X, et al. Curr Chem Genomics. 2012;6:38-47. doi: 10.2174/1875397301206010038. Epub 2012 Sep 20. Curr Chem Genomics. 2012. PMID: 23115612 Free PMC article. - Opening up new fronts in the fight against cholesterol.
Debose-Boyd RA, Horton JD. Debose-Boyd RA, et al. Elife. 2013 Apr 9;2:e00663. doi: 10.7554/eLife.00663. Elife. 2013. PMID: 23580362 Free PMC article. - 3D heterospecies spheroids of pancreatic stroma and cancer cells demonstrate key phenotypes of pancreatic ductal adenocarcinoma.
Liu X, Gündel B, Li X, Liu J, Wright A, Löhr M, Arvidsson G, Heuchel R. Liu X, et al. Transl Oncol. 2021 Jul;14(7):101107. doi: 10.1016/j.tranon.2021.101107. Epub 2021 May 1. Transl Oncol. 2021. PMID: 33946033 Free PMC article. - High-dose atorvastatin causes a rapid sustained increase in human serum PCSK9 and disrupts its correlation with LDL cholesterol.
Welder G, Zineh I, Pacanowski MA, Troutt JS, Cao G, Konrad RJ. Welder G, et al. J Lipid Res. 2010 Sep;51(9):2714-21. doi: 10.1194/jlr.M008144. Epub 2010 Jun 5. J Lipid Res. 2010. PMID: 20525997 Free PMC article. Clinical Trial.
References
- Naureckiene S., et al. Functional characterization of Narc 1, a novel proteinase related to proteinase K. Arch. Biochem. Biophys. 2003;420:55–67. - PubMed
- Benjannet S., et al. NARC-1/PCSK9 and its natural mutants: zymogen cleavage and effects on the low density lipoprotein (LDL) receptor and LDL cholesterol. J. Biol. Chem. 2004;279:48865–48875. - PubMed
- Abifadel M., et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. . Nat. Genet. 2003;34:154–156. - PubMed
- Sun X.-M., et al. Evidence for effect of mutant PCSK9 on apolipoprotein B secretion as the cause of unusually severe dominant hypercholesterolaemia. Hum. Mol. Genet. 2005;14:1161–1169. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous