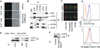Cutting edge: CD4 is the receptor for the tick saliva immunosuppressor, Salp15 - PubMed (original) (raw)
Cutting edge: CD4 is the receptor for the tick saliva immunosuppressor, Salp15
Renu Garg et al. J Immunol. 2006.
Abstract
Salp15 is an Ixodes scapularis salivary protein that inhibits CD4+ T cell activation through the repression of TCR ligation-triggered calcium fluxes and IL-2 production. We show in this study that Salp15 binds specifically to the CD4 coreceptor on mammalian host T cells. Salp15 specifically associates through its C-terminal residues with the outermost two extracellular domains of CD4. Upon binding to CD4, Salp15 inhibits the subsequent TCR ligation-induced T cell signaling at the earliest steps including tyrosine phosphorylation of the Src kinase Lck, downstream effector proteins, and lipid raft reorganization. These results provide a molecular basis to understanding the immunosuppressive activity of Salp15 and its specificity for CD4+ T cells.
Conflict of interest statement
Disclosures
The authors have no financial conflict of interest.
Figures
FIGURE 1
Salp15 binds to CD4. A, Left panels, Analysis of His-tagged Salp15-488 binding to CD4+ and CD8+ T cells by confocal microscopy compared with unstained CD4+ T cells (control). The panels on the right show the same field under brightfield microscopy. B, Murine primary CD4+T cells lysates containing His-tagged Salp15 were immunoprecipitated (IP) using anti-His, anti-CD3ε, anti-CD28, anti-CD4, anti-TCRβ, or IgG (control). The immunoprecipitate was immunoblotted (IB) using anti-His, anti-CD4, anti-CD3ε, anti-TCRβ, and anti-CD28 Abs. C, Colocalization of anti-CD4 staining and His-tagged Salp15-488 binding on naive and stimulated CD4+ T cells is indicated by the yellow color in the merged confocal micrograph. D, Flow cytometric analysis of CD4 expression using PE-Cy5-labeled anti-CD4 (CD4PE-Cy5) (upper panel) and His-tagged Salp15-488 binding (lower panel) in 3T3 (red) and 3T3-CD4 (blue) cells. E, HeLa and HeLa-CD4 cell lysates containing His-tagged Salp15 were immunoprecipitated using anti-His Ab. The immunoprecipitates were subjected to immunoblotting using anti-CD4 and anti-His Abs. The reciprocal immunoprecipitation from a HeLa-CD4 cell lysate was performed using anti-CD4 or IgG followed by immunoblotting with anti-CD4 or anti-His Abs. F, Immunoprecipitation from HeLa-CD4 cell lysate containing either His-tagged TR-Salp13 (control) or His-tagged Salp15 using an anti-His Ab followed by immunoblotting with anti-CD4 or anti-His Abs.
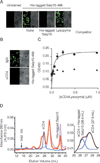
FIGURE 2
Salp15 binds to the extracellular domains of CD4. A, Unlabeled Salp15 but not lysozyme pretreatment blocks His-tagged Salp15-488 binding to HeLa-CD4 cells. Control represents HeLa-CD4 cells in the absence of His-tagged Salp15-488. Right panel, The brightfield of the image on the left. B, Preincubation of HeLa-CD4 cells with a polyclonal anti-CD4 Ab abolishes His-tagged Salp15-488 binding compared with control IgG pretreatment. C, His-tagged Salp15 (0.4 µM) was incubated with increasing amounts of immobilized sCD4 (D1D2, ■) or lysozyme (▲) in a microtiter assay showing saturable binding. The results are expressed as mean ± SE of three independent experiments. D, Elution profiles of sCD4 (D1–D4) (blue), His-tagged Salp15 (pink), and His-tagged Salp15-sCD4 (red) from Superdex-200 gel filtration columns (left panel). The Gaussian deconvolution of His-tagged Salp15-sCD4 and sCD4 peaks is shown on the right panel. The results shown are representative of three to five individual experiments performed.
FIGURE 3
The C-terminal peptide of Salp15 binds CD4. A, Binding of His-tagged Salp15 (5 µg) and overlapping synthetic peptides of Salp15 (0.5 µg) to sCD4 (D1–D2). B, Increasing concentrations of P11 (■) but not P8 (▲) show saturable binding to sCD4 (D1–D2). C, Competition of increasing concentrations of free P11 with immobilized P11 (50 nmol) for binding to sCD4 (D1–D2). D, Competition of P11 (50 nmol) binding to sCD4 (D1–D2) by increasing concentrations of His-tagged Salp15. Control represents P11 binding in the absence of Salp15. The results are expressed as mean ± SE of at least three independent experiments.
FIGURE 4
Salp15 inhibits early steps during T cell activation. A, Western blots showing the increase in tyrosine phosphorylation of Lck at the residue 505, and decreased phosphorylation of Lck at Tyr394, Zap70 at Tyr319, and PLCγ1 at Tyr783 in anti-CD3/CD28-stimulated mouse CD4+ T cells in the presence or absence of His-tagged Salp15. B, Representative immunofluorescence micrographs showing CTB594 staining in CD4+ and CD8+ T cells stimulated with anti-CD3/CD28 in the presence or absence of His-tagged Salp15.
Similar articles
- T-cell signaling pathways inhibited by the tick saliva immunosuppressor, Salp15.
Juncadella IJ, Garg R, Ananthnarayanan SK, Yengo CM, Anguita J. Juncadella IJ, et al. FEMS Immunol Med Microbiol. 2007 Apr;49(3):433-8. doi: 10.1111/j.1574-695X.2007.00223.x. Epub 2007 Mar 2. FEMS Immunol Med Microbiol. 2007. PMID: 17343683 - Salp15, an ixodes scapularis salivary protein, inhibits CD4(+) T cell activation.
Anguita J, Ramamoorthi N, Hovius JW, Das S, Thomas V, Persinski R, Conze D, Askenase PW, Rincón M, Kantor FS, Fikrig E. Anguita J, et al. Immunity. 2002 Jun;16(6):849-59. doi: 10.1016/s1074-7613(02)00325-4. Immunity. 2002. PMID: 12121666 - Two novel Salp15-like immunosuppressant genes from salivary glands of Ixodes persulcatus Schulze tick.
Mori A, Konnai S, Yamada S, Hidano A, Murase Y, Ito T, Takano A, Kawabata H, Onuma M, Ohashi K. Mori A, et al. Insect Mol Biol. 2010 Jun 1;19(3):359-65. doi: 10.1111/j.1365-2583.2010.00994.x. Epub 2010 Feb 26. Insect Mol Biol. 2010. PMID: 20201978 - Salp15, a Multifunctional Protein From Tick Saliva With Potential Pharmaceutical Effects.
Wen S, Wang F, Ji Z, Pan Y, Jian M, Bi Y, Zhou G, Luo L, Chen T, Li L, Ding Z, Abi ME, Liu A, Bao F. Wen S, et al. Front Immunol. 2020 Jan 10;10:3067. doi: 10.3389/fimmu.2019.03067. eCollection 2019. Front Immunol. 2020. PMID: 31998324 Free PMC article. Review. - The immunosuppresive tick salivary protein, Salp15.
Juncadella IJ, Anguita J. Juncadella IJ, et al. Adv Exp Med Biol. 2009;666:121-31. doi: 10.1007/978-1-4419-1601-3_10. Adv Exp Med Biol. 2009. PMID: 20054980 Review.
Cited by
- Changing the Recipe: Pathogen Directed Changes in Tick Saliva Components.
Pham M, Underwood J, Oliva Chávez AS. Pham M, et al. Int J Environ Res Public Health. 2021 Feb 12;18(4):1806. doi: 10.3390/ijerph18041806. Int J Environ Res Public Health. 2021. PMID: 33673273 Free PMC article. Review. - A deep insight into the sialotranscriptome of the gulf coast tick, Amblyomma maculatum.
Karim S, Singh P, Ribeiro JM. Karim S, et al. PLoS One. 2011;6(12):e28525. doi: 10.1371/journal.pone.0028525. Epub 2011 Dec 21. PLoS One. 2011. PMID: 22216098 Free PMC article. - A novel sphingomyelinase-like enzyme in Ixodes scapularis tick saliva drives host CD4 T cells to express IL-4.
Alarcon-Chaidez FJ, Boppana VD, Hagymasi AT, Adler AJ, Wikel SK. Alarcon-Chaidez FJ, et al. Parasite Immunol. 2009 Apr;31(4):210-9. doi: 10.1111/j.1365-3024.2009.01095.x. Parasite Immunol. 2009. PMID: 19292772 Free PMC article. - Preferential protection of Borrelia burgdorferi sensu stricto by a Salp15 homologue in Ixodes ricinus saliva.
Hovius JW, Schuijt TJ, de Groot KA, Roelofs JJ, Oei GA, Marquart JA, de Beer R, van 't Veer C, van der Poll T, Ramamoorthi N, Fikrig E, van Dam AP. Hovius JW, et al. J Infect Dis. 2008 Oct 15;198(8):1189-97. doi: 10.1086/591917. J Infect Dis. 2008. PMID: 18752445 Free PMC article. - Reviewing molecular adaptations of Lyme borreliosis spirochetes in the context of reproductive fitness in natural transmission cycles.
Tsao JI. Tsao JI. Vet Res. 2009 Mar-Apr;40(2):36. doi: 10.1051/vetres/2009019. Epub 2009 Apr 16. Vet Res. 2009. PMID: 19368764 Free PMC article. Review.
References
- Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP. Lyme disease-a tick-borne spirochetosis? Science. 1982;216:1317–1319. - PubMed
- Anguita J, Ramamoorthi N, Hovius JW, Das S, Thomas V, Persinski R, Conze D, Askenase PW, Rincon M, Kantor FS, Fikrig E. Salp15, an Ixodes scapularis salivary protein, inhibits CD4+ T cell activation. Immunity. 2002;16:849–859. - PubMed
- Ferreira BR, Silva JS. Saliva of Rhipicephalus sanguineus tick impairs T cell proliferation and IFN-γ-induced macrophage microbicidal activity. Vet. Immunol. Immunopathol. 1998;64:279–293. - PubMed
- Kopecky J, Kuthejlova M. Suppressive effect of Ixodes ricinus salivary gland extract on mechanisms of natural immunity in vitro. Parasite Immunol. 1998;20:169–174. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous