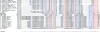A peptidoglycan hydrolase motif within the mycobacteriophage TM4 tape measure protein promotes efficient infection of stationary phase cells - PubMed (original) (raw)
A peptidoglycan hydrolase motif within the mycobacteriophage TM4 tape measure protein promotes efficient infection of stationary phase cells
Mariana Piuri et al. Mol Microbiol. 2006 Dec.
Abstract
The predominant morphotype of mycobacteriophage virions has a DNA-containing capsid attached to a long flexible non-contractile tail, features characteristic of the Siphoviridae. Within these phage genomes the tape measure protein (tmp) gene can be readily identified due to the well-established relationship between the length of the gene and the length of the phage tail--because these phages typically have long tails, the tmp gene is usually the largest gene in the genome. Many of these mycobacteriophage Tmp's contain small motifs with sequence similarity to host proteins. One of these motifs (motif 1) corresponds to the Rpf proteins that have lysozyme activity and function to stimulate growth of dormant bacteria, while the others (motifs 2 and 3) are related to proteins of unknown function, although some of the related proteins of the host are predicted to be involved in cell wall catabolism. We show here that motif 3-containing proteins have peptidoglycan-hydrolysing activity and that while this activity is not required for phage viability, it facilitates efficient infection and DNA injection into stationary phase cells. Tmp's of mycobacteriophages may thus have acquired these motifs in order to avoid a selective disadvantage that results from changes in peptidoglycan in non-growing cells.
Figures
Fig. 1
Amino acid sequence alignment of proteins related to Tmp mt3. Amino acid sequence alignment of proteins containing the ProDom domain that corresponds to mt3 using MultAlign. Omega gp34 and Che8 gp14 were included in the alignment using
clustalx
. Red letters, high consensus (90%); blue letters, low consensus (50%); ! is anyone of IV; # is anyone of NDQE. For Mycobacterial and Mycobacteriophages proteins the correspondent gene name is also shown. The conserved W residue is highlighted.
Fig. 2
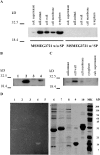
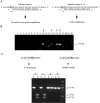
Localization and biochemical activities of MSMEG3721/MSMEG0642. A. Western blot analysis of MSMEG3721 with or without the putative SP overexpressed in M. smegmatis using an extrachromosomal plasmid (pMP2 and pMP1 respectively). Samples of cell fractions are indicated. B. MSMEG3721 gene containing the putative SP was cloned under control of an acetamidase promoter in an integrative vector (pMP4). After integration in M. smegmatis, cells were grown in TH9 containing succinate with and without the acetamide inducer. Proteins were extracted from M. smegmatis cells transformed with pJL37 (empty vector control; lane 1), pMP2 (control of expression w/out SP; lane 2), pMP4 (grown in the absence of acetamide; lane 3), pMP4 (grown in the presence of acetamide; lane 4). C. Cell localization of MSMEG3721 w/SP overexpressed from pMP4 in the presence of acetamide. In all cases proteins were detected in the indicated cell fractions using an α-MSMEG3721 polyclonal antiserum. D. Partially purified protein preparations were separated by SDS-PAGE (right panel) or separated and analysed in a zymogram (left panel) for peptidoglycan-hydrolysing activity. Lanes 1 and 6, MSMEG3721 recombinant protein (10 μg); 2 and 7 MSMEG0642 recombinant protein (10 μg); lanes 3 and 8, mock purification using E. coli cells transformed with pET-28a (empty vector); lanes 4 and 9 lysozyme (5 μg) used as positive control; lanes 5 and 10, β-casein (10 μg) used as a negative control.
Fig. 3
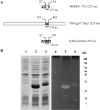
Construction of a hybrid protein carrying TM4 Tmp mt3. A. Schematic representation of TM4 gp17 Tmp and MSMEG3721 showing the position of mt3. A hybrid protein was created by replacing mt3 in MSMEG3721 with the motif present in the Tmp of TM4. B. E. coli cell extracts expressing the indicated proteins were separated by SDS-PAGE (left panel) or separated and analysed in a zymogram for peptidoglycan-hydrolysing activity (right panel). Lanes 1 and 4, extract of E. coli cells transformed with pET-28a (empty vector); lanes 2 and 5, E. coli cell extracts overexpressing MSMEG3721; lanes 3 and 6, E. coli cell extracts overexpressing the hybrid protein.
Fig. 4
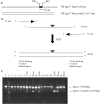
Construction of TM4 deletion mutant lacking Tmp mt3. A. Schematic representation of TM4 gp17 Tmp showing the position of mt3 and the expected product after its deletion. B. Schematic representation of the PCR method used to increase the length of homology in the targeting vector. A 200 bp targeting fragment was obtained from a 100-mer oligonucleotide with 50 bases of homology to each side of the deletion and two 72-mers to generate a 342 bp deletion in TM4 gene 17 (adapted from Swaminathan et al., 2001). C. Identification of mutant clones by PCR. Phasmids extracted from pools of cells recovered without selection (approximately three cells/pool) were analysed by PCR using primers that distinguish wild-type and mutant forms of gene 17 and the products analysed by agarose gel electrophoresis; the amplicon obtained from wt gene 17 is 939 bp and from gene Δ_17_ mt3 is 597 bp. Lanes 3–20 contain 18 representative pools and those containing the phAE87Δ_17_mt3 mutant are marked with an arrow; lane 1 is a parental phAE87 control template and lane 2 is a DNA size marker. Of the total 93 pools tested, 23 contained at least one deletion derivative, and we estimate that the frequency of mutants in the recovered cells is about one recombinant per 12 electroporated cells.
Fig. 5
Construction of a point mutant in TM4 Tmp mt3. To construct a point mutation in the shuttle phasmid phAE87, two complementary single-strand oligonucleotides (100-mer) containing a T→G substitution that changes a TGG for a GGG codon (W853G) in gene 17 were electroporated into an E. coli recombineering strain and pools of recovered cells screened first by allele-specific PCR amplification (MAMA-PCR) (Cha et al., 1992), and then by diagnostic restriction digestion. A. Schematic representation of MAMA-PCR. A detection primer (reverse primer) with a two-base mismatch at the 3′ does not efficiently amplify the wild-type sequence under the PCR conditions used (see Experimental procedures), although the same primer anneals with the mutated sequence and effectively amplifies a 568 bp product. B. PCR to identify positive clones after recombineering. Pools of recombineering products (approximately five cells/pool) were analysed by MAMA-PCR and the products analysed by agarose gel electrophoresis. Lanes 2–19 contain representative pools, and those containing phasmids with the desired single base substitution T→G (phAE87_17_W853G) are indicated with an arrow. Lane 1 contains parent control DNA and lane 20 is a DNA marker. Of the 93 pools tested, 25 contained at least one mutant derivative, corresponding to a frequency of approximately one recombinant per 20 electroporated cells. C. Diagnostic restriction analysis of phAE87_17_W853G. Positive recombineering pools were used to transform XL-Blue cells and individual recovered phasmids used as substrates in a second round of MAMA-PCR. Independently recovered clones were tested by amplification of a 939 bp fragment containing the mutant region of TM4 gene 17, followed by digestion with Pvu I. The T→G substitution generates a new Pvu I site that is absent in the original sequence so that digestion generates 409 bp and 528 bp fragments. Uncut (U) and cut (C) products of two positive (#1 and 2) and two negative (#3 and 4) clones are shown.
Fig. 6
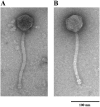
phAE87Δ_17_mt3 is viable but has shorter tails that its parent phage. Electron micrographs of representative particles of phAE87 (A) and phAE87Δ_17_mt3 (B). The average tail length is 200 ± 10 nm for phAE87 and 180 ± 6 for phAE87Δ_17_mt3. The tail lengths of 30 individual phage particles for each phage were measured.
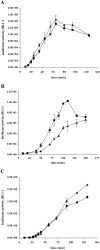
Fig. 7
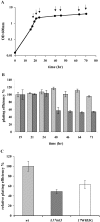
Plating efficiency of wt and mutant phages using cells from different growth phases. A. Growth curve of M. smegmatis mc2155 at 37°C in 7H9 + ADC. The arrows indicate the time points when cells were removed and used in a plaque assay with phAE87 and phAE87Δ_17_mt3. B. Light grey bars correspond to phAE87 and dark grey bars to phAE87Δ_17_mt3. Plating efficiency was calculated relative to the phage titer at 30°C using cells in late exponential phase of growth (19 h). C. Light grey bar corresponds to phAE87, dark grey bar to phAE87Δ_17_mt3 and white bar to phAE87_17_W853G. Relative plating efficiencies were calculated by comparing the phage titer at 30°C using cells in stationary phase (48 h) relative to the phage titer at 30°C using cells in late exponential phase of growth.
Fig. 8
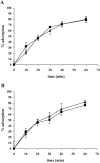
Adsorption assay. Adsorption of phAE8_7_ (▪) and phAE87Δ_17_mt3 (▴). A. Adsorption to M. smegmatis mc2155 cells from exponential phase. B. Adsorption to cells from stationary phase resuspended in spent medium.
Fig. 9
Kinetics of luciferase activity. Kinetics of luciferase activity (relative luciferase units, RLU) after infection with phAE87::FFlux (▪) and phAE87Δ_17_mt3::FFlux (▴). A. Exponential phase cells. B. Stationary phase cells resuspended in spent medium. C. Stationary phase cells resuspended in fresh medium.
Similar articles
- Tape measure protein having MT3 motif facilitates phage entry into stationary phase cells of Mycobacterium tuberculosis.
Dusthackeer A, Hassan VN, Kumar V. Dusthackeer A, et al. Comput Biol Chem. 2008 Oct;32(5):367-9. doi: 10.1016/j.compbiolchem.2008.03.016. Epub 2008 Apr 8. Comput Biol Chem. 2008. PMID: 18479969 - Understanding the role of the lysozyme-like domain of D29 mycobacteriophage-encoded endolysin in host cell lysis and phage propagation.
Joshi H, Nair G, Gangakhedkar R, Jain V. Joshi H, et al. Microbiology (Reading). 2019 Sep;165(9):1013-1023. doi: 10.1099/mic.0.000831. Microbiology (Reading). 2019. PMID: 31264955 - Gp29 LysA of mycobacteriophage TM4 can hydrolyze peptidoglycan through an N-acetyl-muramoyl-L-alanine amidase activity.
Urdániz E, Martín M, Payaslián F, Defelipe LA, Dodes M, Martinez M, Alzari PM, Cabrera G, Martí MA, Piuri M. Urdániz E, et al. Biochim Biophys Acta Proteins Proteom. 2022 Feb 1;1870(2):140745. doi: 10.1016/j.bbapap.2021.140745. Epub 2021 Dec 11. Biochim Biophys Acta Proteins Proteom. 2022. PMID: 34906734 - Mycobacteriophage Lysis Enzymes: Targeting the Mycobacterial Cell Envelope.
Catalão MJ, Pimentel M. Catalão MJ, et al. Viruses. 2018 Aug 14;10(8):428. doi: 10.3390/v10080428. Viruses. 2018. PMID: 30110929 Free PMC article. Review. - On the nature of mycobacteriophage diversity and host preference.
Jacobs-Sera D, Marinelli LJ, Bowman C, Broussard GW, Guerrero Bustamante C, Boyle MM, Petrova ZO, Dedrick RM, Pope WH; Science Education Alliance Phage Hunters Advancing Genomics And Evolutionary Science Sea-Phages Program; Modlin RL, Hendrix RW, Hatfull GF. Jacobs-Sera D, et al. Virology. 2012 Dec 20;434(2):187-201. doi: 10.1016/j.virol.2012.09.026. Epub 2012 Oct 22. Virology. 2012. PMID: 23084079 Free PMC article. Review.
Cited by
- Mycobacteriophage Lysin B is a novel mycolylarabinogalactan esterase.
Payne K, Sun Q, Sacchettini J, Hatfull GF. Payne K, et al. Mol Microbiol. 2009 Aug;73(3):367-81. doi: 10.1111/j.1365-2958.2009.06775.x. Epub 2009 Jun 22. Mol Microbiol. 2009. PMID: 19555454 Free PMC article. - Genome sequence and characterization of the Tsukamurella bacteriophage TPA2.
Petrovski S, Seviour RJ, Tillett D. Petrovski S, et al. Appl Environ Microbiol. 2011 Feb;77(4):1389-98. doi: 10.1128/AEM.01938-10. Epub 2010 Dec 23. Appl Environ Microbiol. 2011. PMID: 21183635 Free PMC article. - An intramolecular cross-talk in D29 mycobacteriophage endolysin governs the lytic cycle and phage-host population dynamics.
Nair G, Jain V. Nair G, et al. Sci Adv. 2024 Feb 9;10(6):eadh9812. doi: 10.1126/sciadv.adh9812. Epub 2024 Feb 9. Sci Adv. 2024. PMID: 38335296 Free PMC article. - The Caulobacter crescentus phage phiCbK: genomics of a canonical phage.
Gill JJ, Berry JD, Russell WK, Lessor L, Escobar-Garcia DA, Hernandez D, Kane A, Keene J, Maddox M, Martin R, Mohan S, Thorn AM, Russell DH, Young R. Gill JJ, et al. BMC Genomics. 2012 Oct 10;13:542. doi: 10.1186/1471-2164-13-542. BMC Genomics. 2012. PMID: 23050599 Free PMC article. - Biodiversity of Streptococcus thermophilus Phages in Global Dairy Fermentations.
Lavelle K, Martinez I, Neve H, Lugli GA, Franz CMAP, Ventura M, Bello FD, Sinderen DV, Mahony J. Lavelle K, et al. Viruses. 2018 Oct 22;10(10):577. doi: 10.3390/v10100577. Viruses. 2018. PMID: 30360457 Free PMC article.
References
- Atrih A, Foster SJ. The role of peptidoglycan structure and structural dynamics during endospore dormancy and germination. Antonie Van Leeuwenhoek. 1999;75:299–307. - PubMed
- Bardarov S, Bardarov S, Jr, Pavelka MS, Jr, Sambandamurthy V, Larsen M, Tufariello J, et al. Specialized transduction: an efficient method for generating marked and unmarked targeted gene disruptions in Mycobacterium tuberculosis, M. bovis BCG and M. smegmatis. Microbiology. 2002;148:3007–3017. - PubMed
- Barsom EK, Hatfull GF. Characterization of Mycobacterium smegmatis gene that confers resistance to phages L5 and D29 when overexpressed. Mol Microbiol. 1996;21:159–170. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources