Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction - PubMed (original) (raw)
. 2006 Nov 27;203(12):2673-82.
doi: 10.1084/jem.20061775. Epub 2006 Nov 6.
Ayako Suematsu, Kazuo Okamoto, Akira Yamaguchi, Yasuyuki Morishita, Yuho Kadono, Sakae Tanaka, Tatsuhiko Kodama, Shizuo Akira, Yoichiro Iwakura, Daniel J Cua, Hiroshi Takayanagi
Affiliations
- PMID: 17088434
- PMCID: PMC2118166
- DOI: 10.1084/jem.20061775
Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction
Kojiro Sato et al. J Exp Med. 2006.
Abstract
In autoimmune arthritis, traditionally classified as a T helper (Th) type 1 disease, the activation of T cells results in bone destruction mediated by osteoclasts, but how T cells enhance osteoclastogenesis despite the anti-osteoclastogenic effect of interferon (IFN)-gamma remains to be elucidated. Here, we examine the effect of various Th cell subsets on osteoclastogenesis and identify Th17, a specialized inflammatory subset, as an osteoclastogenic Th cell subset that links T cell activation and bone resorption. The interleukin (IL)-23-IL-17 axis, rather than the IL-12-IFN-gamma axis, is critical not only for the onset phase, but also for the bone destruction phase of autoimmune arthritis. Thus, Th17 is a powerful therapeutic target for the bone destruction associated with T cell activation.
Figures
Figure 1.
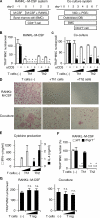
Effects of Th1, Th2, and T reg cells on in vitro osteoclastogenesis. (A) Schematics of two culture systems for osteoclast differentiation and Th cell addition. In the RANKL–M-CSF system, mouse nonadherent BMCs were stimulated with M-CSF for 2 d and adherent cells were used as BMMs. After BMMs were stimulated with recombinant RANKL and M-CSF for 3 d, the formation of TRAP+ MNCs was analyzed. In the co-culture system, BMCs were co-cultured with osteoblasts stimulated with VitD3 and PGE2, and the formation of TRAP+ MNCs was observed 7 d after the addition of BMCs. (B) Inhibitory effects of Th1 and Th2 cells on TRAP+ MNC formation in the RANKL–M-CSF system. Th cells (4,000 or 20,000 cells/ml) were added at the same time as RANKL (day 0) with (black bars) or without (white bars) anti-CD3 mAb. n.d., not detected. (C) Inhibitory effects of Th1 and Th2 cells on TRAP+ MNC formation in the co-culture system. The same number of T cells as in B was added 2 d after BMC addition (day 2). (D) Microphotographs of the in vitro osteoclast formation systems in the presence of Th1 or Th2 cells (20,000 cells/ml) with anti-CD3 mAb (TRAP staining). (E) Cytokine profile of culture supernatants in the presence of Th cells and 1 μg/ml of soluble anti-CD3 mAb (the RANKL–M-CSF system on day 2). Without restimulation by anti-CD3 mAb, cytokine production was much less than this result and was difficult to detect after 2-d culture with osteoclast precursor cells (not depicted). (F) Effects of Th1 and Th2 cells (20,000 cells/ml plus anti-CD3 mAb) on WT or IFN-γ receptor–deficient (Ifngr1 −/−) osteoclast precursor cells. (G) Effects of isolated CD4+CD25+ T reg cells (4,000 or 20,000 cells/ml plus anti-CD3 mAb) on osteoclastogenesis in vitro. n.s., not significantly different. The survival of a considerable number of T reg cells after 3 d was confirmed by CFSE staining (not depicted).
Figure 2.
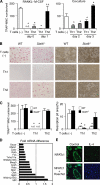
Formation of multinuclear cells with no bone-resorbing activity induced by Th2 cells and IL-4. (A) Inhibitory effects of Th1 and Th2 cells on osteoclastogenesis are reduced when T cells are added 1 d later. Th cells (20,000 cells/ml plus anti-CD3 mAb) were added on days 0 (at the same time as RANKL, gray bars) or 1 (black bars) to the RANKL–M-CSF system and on days 2 (2 d after BMC addition, gray bars) or 3 (black bars) to the co-culture system. (B) Microphotographs and (C) quantification of in vitro osteoclast formation (left, TRAP staining) and resorption pit formation (right). Th1 and Th2 cells (20,000 cells/ml plus anti-CD3 mAb) were added to WT or Stat6 −/− osteoclast precursor cells on day 1. (D) Effect of IL-4 on mRNA expression of osteoclast-related genes in osteoclast precursor cells (GeneChip analysis). Osteoclast precursor cells were stimulated by 10 ng/ml IL-4 from day 1 in the RANKL–M-CSF system and harvested on day 3. Fold mRNA difference was calculated by dividing the average difference of the IL-4–treated sample by that of the control sample. The expressions of most of the osteoclast-specific genes are down-regulated. (E) Reduced expression of NFATc1 protein in the cells treated with IL-4. Osteoclast precursor cells were stimulated by 10 ng/ml IL-4 from day 1 in the RANKL–M-CSF system, fixed on day 3, and stained with anti-NFATc1 antibody followed by Alexa Fluoro 488–labeled secondary antibody.
Figure 3.
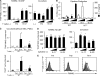
Enhanced osteoclastogenesis by Th17 cells in the co-culture system but not in the RANKL–M-CSF system. (A) Effects of Th1 and Th17 cells on the osteoclast differentiation systems. T cells (4,000 or 20,000 cells/ml plus anti-CD3 mAb) were added on day 1 to the RANKL–M-CSF system and on day 3 to the co-culture system. When the Th17 cells were added 1 d earlier, or in the absence of soluble anti-CD3 mAb, enhancement of osteoclastogenesis was not observed even in the co-culture system (not depicted). (B) Cytokine profile of the culture supernatants obtained on day 3 from the RANKL–M-CSF system in the presence of Th1 and Th17 cells derived from either WT or Il17 −/− mice under the conditions described in A. (C) Effects of Th1 and Th17 cells derived from either WT or Il17 −/− mice on the formation of TRAP+ MNCs or TRAP+ cells in the co-culture system in the absence of VitD3 and PGE2. T cells (20,000 cells/ml plus anti-CD3 mAb) were added on day 3. (D) Effects of recombinant IL-17 and IL-23 (2 or 10 ng/ml) on osteoclastogenesis in vitro. (E) Expression of RANKL on Th subsets. CD4+ T cells cultured in each of the Th conditions for 3 d were restimulated with 1 μg/ml of plate-bound anti-CD3 mAb for 4 h and subjected to flow cytometric analysis using anti-RANKL mAb. Without the restimulation by anti-CD3 mAb, RANKL expression was barely detectable (not depicted).
Figure 4.
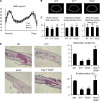
Contribution of IL-17 and IL-23 to the physiological and pathological bone resorption. (A) Bone mineral densities (measured in 20 longitudinal divisions of the femurs), (B) micro-computed tomography (at 10% length above the distal epiphyseal plate), and (C) bone morphometic analyses of WT, Il17 −/−, and Il23a −/− mice at the age of 12 wk. (D) Histological examination of calvarial bones of WT, Il17 −/−, and Il23a −/− mice treated with LPS (hematoxylin and TRAP staining). The degree of bone destruction was analyzed by the number of osteoclasts and the area of the eroded surface (%).
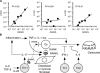
Figure 5.
Regulation of RANKL-mediated osteoclastogenesis by the IL-23–IL-17 axis in the RA synovial tissue. (A) Correlation of the mRNA expression level of RANKL with that of IL23A (p19), IL12A (p35), or IL12B (p40) in the synovium of RA patients. The relative expressions of RANKL, IL23A, IL12A, and IL12B were all standardized using that of GAPDH. (B) Model of Th17-mediated bone destruction in autoimmune arthritis. Th17 cells function as an osteoclastogenic Th cell subset by stimulating local inflammation, inducing RANKL on osteoclastogenesis-supporting cells, and expressing RANKL on themselves and stimulating local inflammation, all of which contribute to an acceleration of osteoclastogenesis. It is notable that RANKL on Th17 cells alone is not sufficient for the induction of osteoclast differentiation (a dotted line). See Discussion for the details. Op, osteoclast precursor cell.
Similar articles
- [Osteoclast differentiation and activation].
Takayanagi H. Takayanagi H. Clin Calcium. 2007 Apr;17(4):484-92. Clin Calcium. 2007. PMID: 17404476 Review. Japanese. - Immune regulation of bone loss by Th17 cells.
Adamopoulos IE, Bowman EP. Adamopoulos IE, et al. Arthritis Res Ther. 2008;10(5):225. doi: 10.1186/ar2502. Epub 2008 Oct 17. Arthritis Res Ther. 2008. PMID: 18983698 Free PMC article. Review. - IL-6-gp130-STAT3 in T cells directs the development of IL-17+ Th with a minimum effect on that of Treg in the steady state.
Nishihara M, Ogura H, Ueda N, Tsuruoka M, Kitabayashi C, Tsuji F, Aono H, Ishihara K, Huseby E, Betz UA, Murakami M, Hirano T. Nishihara M, et al. Int Immunol. 2007 Jun;19(6):695-702. doi: 10.1093/intimm/dxm045. Epub 2007 May 9. Int Immunol. 2007. PMID: 17493959 - The IL-12/IL-23 axis and its role in Th17 cell development, pathology and plasticity in arthritis.
Cornelissen F, van Hamburg JP, Lubberts E. Cornelissen F, et al. Curr Opin Investig Drugs. 2009 May;10(5):452-62. Curr Opin Investig Drugs. 2009. PMID: 19431078 Review. - T cell self-reactivity forms a cytokine milieu for spontaneous development of IL-17+ Th cells that cause autoimmune arthritis.
Hirota K, Hashimoto M, Yoshitomi H, Tanaka S, Nomura T, Yamaguchi T, Iwakura Y, Sakaguchi N, Sakaguchi S. Hirota K, et al. J Exp Med. 2007 Jan 22;204(1):41-7. doi: 10.1084/jem.20062259. Epub 2007 Jan 16. J Exp Med. 2007. PMID: 17227914 Free PMC article.
Cited by
- Gluten-sensitive enteropathy coincides with decreased capability of intestinal T cells to secrete IL-17 and IL-22 in a macaque model for celiac disease.
Xu H, Feely SL, Wang X, Liu DX, Borda JT, Dufour J, Li W, Aye PP, Doxiadis GG, Khosla C, Veazey RS, Sestak K. Xu H, et al. Clin Immunol. 2013 Apr;147(1):40-49. doi: 10.1016/j.clim.2013.02.012. Epub 2013 Feb 28. Clin Immunol. 2013. PMID: 23518597 Free PMC article. - Probiotics ameliorate alveolar bone loss by regulating gut microbiota.
Jia L, Tu Y, Jia X, Du Q, Zheng X, Yuan Q, Zheng L, Zhou X, Xu X. Jia L, et al. Cell Prolif. 2021 Jul;54(7):e13075. doi: 10.1111/cpr.13075. Epub 2021 Jun 7. Cell Prolif. 2021. PMID: 34101283 Free PMC article. - Osteoporosis in postmenopausal women is associated with disturbances in gut microbiota and migration of peripheral immune cells.
Ma Z, Liu Y, Shen W, Yang J, Wang T, Li Y, Ma J, Zhang X, Wang H. Ma Z, et al. BMC Musculoskelet Disord. 2024 Oct 7;25(1):791. doi: 10.1186/s12891-024-07904-1. BMC Musculoskelet Disord. 2024. PMID: 39375626 Free PMC article. - Targeting interleukin-17 in patients with active rheumatoid arthritis: rationale and clinical potential.
Kellner H. Kellner H. Ther Adv Musculoskelet Dis. 2013 Jun;5(3):141-52. doi: 10.1177/1759720X13485328. Ther Adv Musculoskelet Dis. 2013. PMID: 23858337 Free PMC article. - Inflammatory arthritis increases mouse osteoclast precursors with myeloid suppressor function.
Charles JF, Hsu LY, Niemi EC, Weiss A, Aliprantis AO, Nakamura MC. Charles JF, et al. J Clin Invest. 2012 Dec;122(12):4592-605. doi: 10.1172/JCI60920. Epub 2012 Nov 1. J Clin Invest. 2012. PMID: 23114597 Free PMC article.
References
- Walsh, M.C., N. Kim, Y. Kadono, J. Rho, S.Y. Lee, J. Lorenzo, and Y. Choi. 2006. Osteoimmunology: interplay between the immune system and bone metabolism. Annu. Rev. Immunol. 24:33–63. - PubMed
- Takayanagi, H. 2005. Inflammatory bone destruction and osteoimmunology. J. Periodontal Res. 40:287–293. - PubMed
- Sato, K., and H. Takayanagi. 2006. Osteoclasts, rheumatoid arthritis, and osteoimmunology. Curr. Opin. Rheumatol. 18:419–426. - PubMed
- Theill, L.E., W.J. Boyle, and J.M. Penninger. 2002. RANK-L and RANK: T cells, bone loss, and mammalian evolution. Annu. Rev. Immunol. 20:795–823. - PubMed
- Teitelbaum, S.L., and F.P. Ross. 2003. Genetic regulation of osteoclast development and function. Nat. Rev. Genet. 4:638–649. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases