The marine viromes of four oceanic regions - PubMed (original) (raw)
doi: 10.1371/journal.pbio.0040368.
Ben Felts, Mya Breitbart, Peter Salamon, Robert A Edwards, Craig Carlson, Amy M Chan, Matthew Haynes, Scott Kelley, Hong Liu, Joseph M Mahaffy, Jennifer E Mueller, Jim Nulton, Robert Olson, Rachel Parsons, Steve Rayhawk, Curtis A Suttle, Forest Rohwer
Affiliations
- PMID: 17090214
- PMCID: PMC1634881
- DOI: 10.1371/journal.pbio.0040368
The marine viromes of four oceanic regions
Florent E Angly et al. PLoS Biol. 2006 Nov.
Abstract
Viruses are the most common biological entities in the marine environment. There has not been a global survey of these viruses, and consequently, it is not known what types of viruses are in Earth's oceans or how they are distributed. Metagenomic analyses of 184 viral assemblages collected over a decade and representing 68 sites in four major oceanic regions showed that most of the viral sequences were not similar to those in the current databases. There was a distinct "marine-ness" quality to the viral assemblages. Global diversity was very high, presumably several hundred thousand of species, and regional richness varied on a North-South latitudinal gradient. The marine regions had different assemblages of viruses. Cyanophages and a newly discovered clade of single-stranded DNA phages dominated the Sargasso Sea sample, whereas prophage-like sequences were most common in the Arctic. However most viral species were found to be widespread. With a majority of shared species between oceanic regions, most of the differences between viral assemblages seemed to be explained by variation in the occurrence of the most common viral species and not by exclusion of different viral genomes. These results support the idea that viruses are widely dispersed and that local environmental conditions enrich for certain viral types through selective pressure.
Conflict of interest statement
Competing interests. The authors have declared that no competing interests exist.
Figures
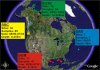
Figure 1. Sampling Sites
The circles represent the sampling locations in the Sargasso Sea (SAR), Gulf of Mexico (GOM), British Columbia (BBC), and the Arctic Ocean. The number of samples taken at each location and combined for sequencing, as well as the date and depth range, are shown in the boxes.
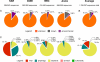
Figure 2. Composition of the Assemblage Genome Sequences as Determined by Similarity to Known DNA and Protein Sequences
(A) The percent of “known” sequences compared to the SEED and environmental databases. A sequence was considered “known” if it had a significant similarity (E < 10−5) to the SEED, else “environmental” if it had a similarity to any environmental database, and else “unknown”. (B) Breakdown of the “known” sequences into viral (both eukaryotic and bacteriophages), prophage, Bacteria, Archaea, or Eukarya.
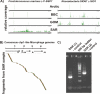
Figure 3. Distribution of Similarities and Assembly Controls
(A) Distribution of similarities between the four metagenome samples to the P. marinus φ P-SSP7 and Roseobacteria SIO67 φ SIO1 genomes (as determined by BLASTN analysis). The green bars represent the average number of sequences averaged over 100 bp windows. (B) Comparison of fragments from the Sargasso Sea metagenome against the consensus ssDNA chp1-like microphage genome. The consensus from this assembly is in the Protocol S1. (C) PCR verification of chp1-like microphages in original SAR sample. PCR primers were designed based on a consensus sequence from the assembly shown in (B). SAR1 is a ~900-bp fragment and SAR2 is a ~1,500-bp fragment.
Figure 4. Types of Phages in the Four Metagenomes
A new version of the Phage Proteomic Tree (left panel) was constructed from 510 complete phage and prophage genomes using the previously described method [23]. The metagenomic sequences were compared to the phage on the Phage Proteomic Tree using TBLASTX, and the colored bars on the right represent significant similarities (_E_-value < 0.0001). Names of prophages are in red and the Prochlorococcus phage genomes are in green. An electronic version of the tree and a FASTA list of phage and prophage genomes used to make the tree are available at the SDSU Center for Universal Microbe Sequencing website (
http://scums.sdsu.edu/phage/Oceans
).
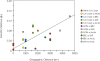
Figure 5. Relationship between Geographic and Genetic Distances of Marine Viral Assemblages
In addition to the four metagenomes sequenced for this study, the previous viral metagenomes from the San Diego area (California coast) were also included in this analysis [10]. There was a significant correlation of 3.28 × 10−5 ΦST / km (Mantel test, Z = −78.9, p < 0.017, r = 0.585).
Figure 6. Monte Carlo Simulation of Cross-Contigs between Metagenomic Samples
(A) For the intersample analysis, the maximum likelihood occurred at 35% fraction permuted and 100% fraction shared. (B) The maximum likelihood was between 0% and 0.5% fraction permuted and 85% and 95 % fraction shared for the intrasample controls.
Comment in
- Metagenomics offers a big-picture view of the diversity and distribution of marine viruses.
Hoff M. Hoff M. PLoS Biol. 2006 Nov;4(11):e406. doi: 10.1371/journal.pbio.0040406. Epub 2006 Nov 7. PLoS Biol. 2006. PMID: 20076501 Free PMC article. No abstract available.
Similar articles
- Oxygen minimum zones harbour novel viral communities with low diversity.
Cassman N, Prieto-Davó A, Walsh K, Silva GG, Angly F, Akhter S, Barott K, Busch J, McDole T, Haggerty JM, Willner D, Alarcón G, Ulloa O, DeLong EF, Dutilh BE, Rohwer F, Dinsdale EA. Cassman N, et al. Environ Microbiol. 2012 Nov;14(11):3043-65. doi: 10.1111/j.1462-2920.2012.02891.x. Epub 2012 Oct 5. Environ Microbiol. 2012. PMID: 23039259 - Diverse and unique viruses discovered in the surface water of the East China Sea.
Wu S, Zhou L, Zhou Y, Wang H, Xiao J, Yan S, Wang Y. Wu S, et al. BMC Genomics. 2020 Jun 26;21(1):441. doi: 10.1186/s12864-020-06861-y. BMC Genomics. 2020. PMID: 32590932 Free PMC article. - Bacteriophage Distributions and Temporal Variability in the Ocean's Interior.
Luo E, Aylward FO, Mende DR, DeLong EF. Luo E, et al. mBio. 2017 Nov 28;8(6):e01903-17. doi: 10.1128/mBio.01903-17. mBio. 2017. PMID: 29184020 Free PMC article. - Virophages to viromes: a report from the frontier of viral oceanography.
Culley AI. Culley AI. Curr Opin Virol. 2011 Jul;1(1):52-7. doi: 10.1016/j.coviro.2011.05.003. Epub 2011 Jun 11. Curr Opin Virol. 2011. PMID: 22440567 Review. - Microbial assemblages for environmental quality assessment: Knowledge, gaps and usefulness in the European Marine Strategy Framework Directive.
Caruso G, La Ferla R, Azzaro M, Zoppini A, Marino G, Petochi T, Corinaldesi C, Leonardi M, Zaccone R, Fonda Umani S, Caroppo C, Monticelli L, Azzaro F, Decembrini F, Maimone G, Cavallo RA, Stabili L, Hristova Todorova N, K Karamfilov V, Rastelli E, Cappello S, Acquaviva MI, Narracci M, De Angelis R, Del Negro P, Latini M, Danovaro R. Caruso G, et al. Crit Rev Microbiol. 2016 Nov;42(6):883-904. doi: 10.3109/1040841X.2015.1087380. Epub 2015 Nov 20. Crit Rev Microbiol. 2016. PMID: 26585708 Review.
Cited by
- T4-like myovirus community shaped by dispersal and deterministic processes in the South China Sea.
Li H, Liu L, Wang Y, Cai L, He M, Wang L, Hu C, Jiao N, Zhang R. Li H, et al. Environ Microbiol. 2021 Feb;23(2):1038-1052. doi: 10.1111/1462-2920.15290. Epub 2020 Nov 3. Environ Microbiol. 2021. PMID: 33089595 Free PMC article. - Latitudinal variation in virus-induced mortality of phytoplankton across the North Atlantic Ocean.
Mojica KD, Huisman J, Wilhelm SW, Brussaard CP. Mojica KD, et al. ISME J. 2016 Feb;10(2):500-13. doi: 10.1038/ismej.2015.130. Epub 2015 Aug 11. ISME J. 2016. PMID: 26262815 Free PMC article. - Lysogeny and sporulation in Bacillus isolates from the Gulf of Mexico.
Mobberley J, Authement RN, Segall AM, Edwards RA, Slepecky RA, Paul JH. Mobberley J, et al. Appl Environ Microbiol. 2010 Feb;76(3):829-42. doi: 10.1128/AEM.01710-09. Epub 2009 Dec 11. Appl Environ Microbiol. 2010. PMID: 20008174 Free PMC article. - Divide and conquer: enriching environmental sequencing data.
Bergeron A, Belcaid M, Steward GF, Poisson G. Bergeron A, et al. PLoS One. 2007 Sep 5;2(9):e830. doi: 10.1371/journal.pone.0000830. PLoS One. 2007. PMID: 17786202 Free PMC article. - New dimensions of the virus world discovered through metagenomics.
Kristensen DM, Mushegian AR, Dolja VV, Koonin EV. Kristensen DM, et al. Trends Microbiol. 2010 Jan;18(1):11-9. doi: 10.1016/j.tim.2009.11.003. Epub 2009 Nov 26. Trends Microbiol. 2010. PMID: 19942437 Free PMC article.
References
- Weinbauer MG. Ecology of prokaryotic viruses. FEMS Microbiol Rev. 2004;28:127–181. - PubMed
- Wilcox RM, Fuhrman JA. Bacterial viruses in coastal seawater: Lytic rather than lysogenic production. Mar Ecol Prog Ser. 1994;114:35–45.
- Canchaya C, Fournous G, Chibani-Chennoufi S, Dillmann ML, Brussow H. Phage as agents of lateral gene transfer. Curr Opin Microbiol. 2003;6:417–424. - PubMed
- Davis BM, Waldor MK. Mobile genetic elements and bacteria pathogenesis. In: Craig NL, Gragie R, Gellert M, Lambowitz AM, editors. Mobile DNA II. Washington (DC): ASM Press; 2002. pp. 1040–1055.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources