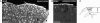Redefining the concept of reactive astrocytes as cells that remain within their unique domains upon reaction to injury - PubMed (original) (raw)
Redefining the concept of reactive astrocytes as cells that remain within their unique domains upon reaction to injury
Ulrika Wilhelmsson et al. Proc Natl Acad Sci U S A. 2006.
Abstract
Reactive astrocytes in neurotrauma, stroke, or neurodegeneration are thought to undergo cellular hypertrophy, based on their morphological appearance revealed by immunohistochemical detection of glial fibrillary acidic protein, vimentin, or nestin, all of them forming intermediate filaments, a part of the cytoskeleton. Here, we used a recently established dye-filling method to reveal the full three-dimensional shape of astrocytes assessing the morphology of reactive astrocytes in two neurotrauma models. Both in the denervated hippocampal region and the lesioned cerebral cortex, reactive astrocytes increased the thickness of their main cellular processes but did not extend to occupy a greater volume of tissue than nonreactive astrocytes. Despite this hypertrophy of glial fibrillary acidic protein-containing cellular processes, interdigitation between adjacent hippocampal astrocytes remained minimal. This work helps to redefine the century-old concept of hypertrophy of reactive astrocytes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Entorhinal cortex lesion triggers reactive gliosis in the hippocampus. Unilateral entorhinal cortex lesion triggers astrocyte activation in the outer and middle molecular layer of the ipsilateral dentate gyrus of the hippocampus (Right, in gray). Antibodies against GFAP visualize bundles of intermediate filaments predominantly found in the soma and the main cellular processes of astrocytes. Four days after unilateral entorhinal cortex lesioning, astrocytes in the molecular layer of the dentate gyrus on the injured side were reactive and all showed greater GFAP immunoreactivity (Center) than the nonreactive astrocytes on the contralateral side (Left). The square in Right denotes the area corresponding to the images in Left and Center. EC, entorhinal cortex. (Scale bar, 25 μm.)
Fig. 2.
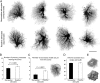
Morphological assessment of reactive and nonreactive astrocytes in the hippocampus. (A) Maximum projections of dye-filled reactive and nonreactive astrocytes in the molecular layer of the dentate gyrus 4 days after entorhinal cortex lesioning. Dye-filling revealed fine spongiform processes in reactive astrocytes comparable with those of nonreactive astrocytes but a greater number of main processes extending from the cell soma of reactive astrocytes. (Scale bar, 25 μm.) (B and C) Quantification of main cellular processes leaving the soma (B) and processes visible 25 μm from the cell soma (C). Thick processes were more numerous in reactive astrocytes than in nonreactive astrocytes. Three-dimensional reconstruction (E) shows that reactive and nonreactive astrocytes access similar volumes of tissue (D; unit y axis 103 μm3). Error bars represent SEM.
Fig. 3.
Electrically induced lesion of the cerebral cortex triggers astrocyte activation in the surrounding tissue. Four days after injury, reactive astrocytes in the cerebral cortex around the lesion show highly increased GFAP immunoreactivity (A and B). (C) Schematic presentation of the injury with the rectangle corresponding to the area shown in A. An asterisk indicates the necrotic area; the dotted line indicates the injury border. CC, corpus callosum. (Scale bar, 100 μm.)
Fig. 4.
Morphological assessment of reactive and nonreactive cortical astrocytes. (A) Maximum projections of dye-filled reactive and nonreactive astrocytes in layer I of cerebral cortex 4 days after cortical lesioning. Dye-filling reveals fine spongiform processes in reactive astrocytes comparable to those of nonreactive astrocytes but a greater number of main processes extending from the cell soma of reactive astrocytes. (Scale bar, 25 μm.) (B and C) Quantification of main cellular processes leaving the soma (B) and processes visible 15 μm from the cell soma (C). Thick processes were more numerous in reactive astrocytes than in nonreactive astrocytes. Three-dimensional reconstruction shows that reactive and nonreactive astrocytes access similar volumes of tissue (D; unit y axis 103 μm3). Error bars represent SEM.
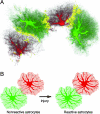
Fig. 5.
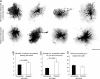
Overlap between astrocyte territories assessed by dye-filling of neighboring cells (Lucifer yellow and Alexa Fluor 568). Maximum projections of astrocytes in the molecular layer of the dentate gyrus on the lesioned and contralateral sides show adjacent astrocyte territories (domains) on three optical sections 4 μm apart. Insets show territories with overlapping areas in yellow. The extent of interdigitation between neighboring astrocytes, both reactive and nonreactive, was limited and most prominent around blood vessels (arrowheads). (Scale bar 25 μm.)
Fig. 6.
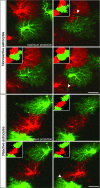
The domains of nonreactive and reactive astrocytes—a concept. (A) Interdigitation of fine cellular processes in a 3D reconstruction of astrocytes in the dentate gyrus. The yellow zone shows the border area where cellular processes of two adjacent astrocytes interdigitate. (B) Reactive astrocytes stay within their domains, but their main cellular processes get thicker, making them visible over a greater distance (illustrated here by the circles).
Similar articles
- Regional difference of reactive astrogliosis following traumatic brain injury revealed by hGFAP-GFP transgenic mice.
Kim WR, Kim JY, Moon Y, Kim HJ, Kim H, Sun W. Kim WR, et al. Neurosci Lett. 2012 Apr 4;513(2):155-9. doi: 10.1016/j.neulet.2012.02.023. Epub 2012 Feb 17. Neurosci Lett. 2012. PMID: 22343312 - Podoplanin: a marker for reactive gliosis in gliomas and brain injury.
Kolar K, Freitas-Andrade M, Bechberger JF, Krishnan H, Goldberg GS, Naus CC, Sin WC. Kolar K, et al. J Neuropathol Exp Neurol. 2015 Jan;74(1):64-74. doi: 10.1097/NEN.0000000000000150. J Neuropathol Exp Neurol. 2015. PMID: 25470350 - A subpopulation of reactive astrocytes at the immediate site of cerebral cortical injury.
Yang HY, Lieska N, Kriho V, Wu CM, Pappas GD. Yang HY, et al. Exp Neurol. 1997 Jul;146(1):199-205. doi: 10.1006/exnr.1997.6518. Exp Neurol. 1997. PMID: 9225753 - The role of astrocytes and complement system in neural plasticity.
Pekny M, Wilhelmsson U, Bogestål YR, Pekna M. Pekny M, et al. Int Rev Neurobiol. 2007;82:95-111. doi: 10.1016/S0074-7742(07)82005-8. Int Rev Neurobiol. 2007. PMID: 17678957 Review. - Role of glial filaments in cells and tumors of glial origin: a review.
Rutka JT, Murakami M, Dirks PB, Hubbard SL, Becker LE, Fukuyama K, Jung S, Tsugu A, Matsuzawa K. Rutka JT, et al. J Neurosurg. 1997 Sep;87(3):420-30. doi: 10.3171/jns.1997.87.3.0420. J Neurosurg. 1997. PMID: 9285609 Review.
Cited by
- Metabolic changes in early poststatus epilepticus measured by MR spectroscopy in rats.
Wu Y, Pearce PS, Rapuano A, Hitchens TK, de Lanerolle NC, Pan JW. Wu Y, et al. J Cereb Blood Flow Metab. 2015 Nov;35(11):1862-70. doi: 10.1038/jcbfm.2015.145. Epub 2015 Jun 24. J Cereb Blood Flow Metab. 2015. PMID: 26104287 Free PMC article. - Neuroprotection Induced by Transplanted CDK5 Knockdown Astrocytes in Global Cerebral Ischemic Rats.
Becerra-Calixto A, Cardona-Gómez GP. Becerra-Calixto A, et al. Mol Neurobiol. 2017 Nov;54(9):6681-6696. doi: 10.1007/s12035-016-0162-2. Epub 2016 Oct 15. Mol Neurobiol. 2017. PMID: 27744570 - GFAP isoforms control intermediate filament network dynamics, cell morphology, and focal adhesions.
Moeton M, Stassen OM, Sluijs JA, van der Meer VW, Kluivers LJ, van Hoorn H, Schmidt T, Reits EA, van Strien ME, Hol EM. Moeton M, et al. Cell Mol Life Sci. 2016 Nov;73(21):4101-20. doi: 10.1007/s00018-016-2239-5. Epub 2016 May 3. Cell Mol Life Sci. 2016. PMID: 27141937 Free PMC article. - Absence of TLR4 reduces neurovascular unit and secondary inflammatory process after traumatic brain injury in mice.
Ahmad A, Crupi R, Campolo M, Genovese T, Esposito E, Cuzzocrea S. Ahmad A, et al. PLoS One. 2013;8(3):e57208. doi: 10.1371/journal.pone.0057208. Epub 2013 Mar 28. PLoS One. 2013. PMID: 23555560 Free PMC article. - Glial Scar-a Promising Target for Improving Outcomes After CNS Injury.
He Y, Liu X, Chen Z. He Y, et al. J Mol Neurosci. 2020 Mar;70(3):340-352. doi: 10.1007/s12031-019-01417-6. Epub 2019 Nov 27. J Mol Neurosci. 2020. PMID: 31776856 Review.
References
- Ullian EM, Sapperstein SK, Christopherson KS, Barres BA. Science. 2001;291:657–661. - PubMed
- Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, Barres BA. Cell. 2005;120:421–433. - PubMed
- Mulligan SJ, MacVicar BA. Nature. 2004;431:195–199. - PubMed
- Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Nat Neurosci. 2003;6:43–50. - PubMed
- Grosche J, Matyash V, Moller T, Verkhratsky A, Reichenbach A, Kettenmann H. Nat Neurosci. 1999;2:139–143. - PubMed
Publication types
MeSH terms
Grants and funding
- RR004050/RR/NCRR NIH HHS/United States
- NS046068/NS/NINDS NIH HHS/United States
- R01 NS046068/NS/NINDS NIH HHS/United States
- NS014718/NS/NINDS NIH HHS/United States
- P41 RR004050/RR/NCRR NIH HHS/United States
- R01 NS014718/NS/NINDS NIH HHS/United States
- CA4084314/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources