Broad-spectrum effects of 4-aminopyridine to modulate amyloid beta1-42-induced cell signaling and functional responses in human microglia - PubMed (original) (raw)
Comparative Study
Broad-spectrum effects of 4-aminopyridine to modulate amyloid beta1-42-induced cell signaling and functional responses in human microglia
Sonia Franciosi et al. J Neurosci. 2006.
Abstract
We investigated the modulating actions of the nonselective K(+) channel blocker 4-aminopyridine (4-AP) on amyloid beta (Abeta(1-42))-induced human microglial signaling pathways and functional processes. Whole-cell patch-clamp studies showed acute application of Abeta(1-42) (5 mum) to human microglia led to rapid expression of a 4-AP-sensitive, non-inactivating outwardly rectifying K(+) current (I(K)). Intracellular application of the nonhydrolyzable analog of GTP, GTPgammaS, induced an outward K(+) current with similar properties to the Abeta(1-42)-induced I(K) including sensitivity to 4-AP (IC(50) = 5 mm). Reverse transcriptase-PCR showed a rapid expression of a delayed rectifier Kv3.1 channel in Abeta(1-42)-treated microglia. Abeta(1-42) peptide also caused a slow, progressive increase in levels of [Ca(2+)](i) (intracellular calcium) that was partially blocked by 4-AP. Chronic exposure of human microglia to Abeta(1-42) led to enhanced p38 mitogen-activated protein kinase and nuclear factor kappaB expression with factors inhibited by 4-AP. Abeta(1-42) also induced the expression and production of the pro-inflammatory cytokines interleukin (IL)-1beta, IL-6, and tumor necrosis factor-alpha, the chemokine IL-8, and the enzyme cyclooxygenase-2; 4-AP was effective in reducing all of these pro-inflammatory mediators. Additionally, toxicity of supernatant from Abeta(1-42)-treated microglia on cultured rat hippocampal neurons was reduced if 4-AP was included with peptide. In vivo, injection of Abeta(1-42) into rat hippocampus induced neuronal damage and increased microglial activation. Daily administration of 1 mg/kg 4-AP was found to suppress microglial activation and exhibited neuroprotection. The overall results suggest that 4-AP modulation of an Abeta(1-42)-induced I(K) (candidate channel Kv3.1) and intracellular signaling pathways in human microglia could serve as a therapeutic strategy for neuroprotection in Alzheimer's disease pathology.
Figures
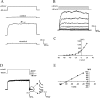
Figure 1.
Aβ1–42 induces an outwardly rectifying current (_I_K) that is attributed to K+. A, A representative recording of the Aβ1–42-induced outward K+ current (_I_K) in response to a depolarizing step in human microglia. The trace is a typical leak current evoked in control solution with a depolarization step to +20 mV from a holding potential of −60 mV. Aβ1–42 (5 μ
m
) elicited a large _I_K with the same step depolarization. The current recovered subsequent to washoff of Aβ1–42. B, _I_K, induced by Aβ1–42 (5 μ
m
), with sequential depolarizing steps applied from −60 mV to a maximum level of +20 mV in 10 mV increments. C, The I_–_V relationship constructed from the pulse protocol shown indicates that the outward current induced by acute Aβ1–42 was outwardly rectifying with a threshold of −40 mV. The figure is a representative recording from one cell. D, Determination of the reversal potential of the _I_K via analysis of tail currents. The protocol for tail current analysis consisted of applying a depolarizing step from −60 to +40 mV, followed by a secondary step to potentials varying from −100 to −20 mV. The resulting tail currents elicited with the secondary steps from −100 to −20 mV in a representative experiment are indicated by arrows. E, An I–V plot of tail current amplitudes versus step potential is shown, and results indicate that the reversal potential of the current is −78 mV, which is close to the equilibrium potential for K+.
Figure 2.
A, _I_K is inhibited by the nonselective K+ channel inhibitor 4-AP. A representative recording from one cell is shown. The first trace is a typical current evoked in control solution with a depolarization step to +20 mV. Aβ1–42 (5 μ
m
) elicited _I_K with the same step depolarization. Application of 4-AP (2 m
m
) in the presence of Aβ1–42 reduced _I_K to 52% of control. The current recovered subsequent to washoff of 4-AP with Aβ1–42 maintained in bath solution. B, Typical profile of the intracellular GTPγS-induced outward current. Intracellular application of GTPγS (10 μ
m
) via the electrode induced an outward current within minutes of rupture of the cell membrane in the whole-cell patch-clamp mode in response to a depolarizing step from −60 to +20 mV. Extracellular application of 4-AP (2 m
m
) reduced the outward K+ current to 65% of the control. Washoff of 4-AP allowed the current to recover. C, Concentration-dependent inhibition of the intracellular GTPγS-induced outward K+ current by 4-AP. 4-AP was applied in the extracellular bath solution, and amplitudes of the GTPγS-induced currents were measured in the presence of 4-AP and normalized to control amplitudes (C; current amplitude before 4-AP application). Results are a summary of the following: n = 4 cells for 1 m
m
; n = 6 cells for 2 m
m
; n = 10 cells for 5 m
m
; n = 4 cells for 10 m
m;
n = 3 cells for 20 m
m
.
Figure 3.
A, A representative RT-PCR experiment of Aβ1–42 (5 μ
m
) treatment for 10 min, 30 min, 1 h, and 2 h on Kv1.1, Kv1.2, Kv1.3, Kv1.5, Kv1.6, Kv2.1, and Kv3.1 channel expression from n = 3 independent experiments. G3PDH served as a reaction standard. B, Summary of relative mRNA levels of Kv channels induced by Aβ1–42. Results are expressed as mean ± SEM from n = 3 independent experiments. One-way ANOVA and the Newman–Keuls multiple comparison test was used to evaluate statistical significance. *p < 0.05 and **p < 0.001, statistical significance from control. C, PTX (100 ng/ml) before treatment (2 h) attenuated the effects of Aβ1–42 (5 μ
m
; 30 min) to increase the expression of Kv3.1.
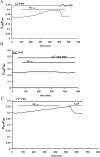
Figure 4.
A, Acute application of Aβ1–42 induces a slow, progressive increase in [Ca2+]i. A representative trace of the increase in [Ca2+]i induced by Aβ1–42 (5 μ
m
) in Ca2+-PSS (n = 21 cells) is shown. Subsequent application of Aβ1–42 in Ca2+-free PSS resulted in an immediate decrease in [Ca2+]i to baseline levels. B, The standard PSS was first exchanged for Ca2+-free PSS. Acute application of Aβ1–42 (5 μ
m
) in Ca2+-free PSS did not elicit an increase in [Ca2+]i (n = 23 cells). C, A representative trace of the effect of 4-AP on the Ca2+ influx pathway induced by Aβ1–42 (n = 26 cells). Subsequent to the slow, progressive increase in [Ca2+]i induced by acute Aβ1–42 (5 μ
m
) in Ca2+ PSS, application of 4-AP (2 m
m
) rapidly decreased [Ca2+]i to baseline levels.
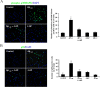
Figure 5.
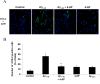
Effects of 4-AP on Aβ1–42-induced p38 MAPK and NF-κB activation. A, Left, Representative photomicrographs of phosphorylated p38 (phospho-p38)-stained microglia. Green and blue indicate staining for phospho-p38 MAPK- and DAPI-positive nuclei, respectively. Under control conditions, little or no phospho-p38 MAPK expression was observed. Aβ1–42 (5 μ
m
) treatment of microglia for 30 min induced an intense expression of phospho-p38 MAPK. Aβ1–42 in the maintained presence of 4-AP (2 m
m
) inhibited expression of phospho-p38 MAPK. Application of 4-AP (2 m
m
) alone had no effect on phospho-p38 MAPK expression. Right, The percentage of phospho-p38 MAPK-positive microglia relative to total cells is shown. Data are means ± SEM from four independent experiments. *p < 0.001, significance compared with control; **p < 0.001, significance compared with Aβ1–42. B, Left, Effects of 4-AP on Aβ1–42-induced NF-κB activation. Representative photomicrographs of p65 (the active subunit of NF-κB)-stained microglia are shown. Green and blue indicate staining for p65- and DAPI-positive nuclei, respectively. Under control conditions, little or no p65 expression was observed. Aβ1–42 (5 μ
m
) treatment of microglia for 8 h induced an intense expression of p65. Aβ1–42 in the maintained presence of 4-AP (2 m
m
) inhibited expression of p65. Application of 4-AP (2 m
m
) alone had no effect on p65 expression. Right, The percentage of p65-positive microglia relative to total cells is shown. Data are means ± SEM from five independent experiments. *p < 0.01, significance compared with control; **p < 0.01, significance compared with Aβ1–42.
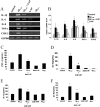
Figure 6.
Effects of 4-AP on Aβ1–42-induced expression and production of pro-inflammatory mediators by human microglia. A, Expression of IL-1β, IL-6, IL-8, TNF-α, and COX-2 were examined in microglia incubated for 8 h with Aβ1–42, 4-AP, Aβ1–42 in the presence of 4-AP (2 m
m
), or medium alone. Stimulation of microglia with vehicle solution or Aβ42–1 (5 μ
m
) alone served as control. The results shown are a representative of seven independent experiments. The expression of G3PDH served as a reaction standard. B, Summary of relative mRNA levels of inflammatory mediators induced by Aβ1–42, 4-AP, and combined Aβ1–42 and 4-AP. Results are expressed as mean ± SEM from n = 7 independent experiments. One-way ANOVA and the Newman–Keuls multiple comparison post-test were performed to evaluate statistical significance. *p < 0.05, statistically significant from control; **p < 0.05, statistically significant from Aβ1–42-stimulated levels. Effects of Aβ1–42, 4-AP, and Aβ1–42 in the maintained presence of 4-AP on pro-inflammatory cytokine secretion by human microglia using ELISA. C–F, Data are mean ± SEM of TNF-α from four independent experiments (C), IL-6 from three independent experiments (D), IL-1β from six independent experiments (E), and IL-8 from four independent experiments (F); each experiment was performed in duplicate. Human microglia were exposed to medium alone, Aβ1–42 (5 μ
m
), 4-AP (2 m
m
), Aβ1–42 in the presence of 4-AP, or Aβ42–1 for 48 h. One-way ANOVA and the Newman–Keuls multiple comparison post-test were performed to evaluate statistical significance. *p < 0.001, statistical significance from control; **p < 0.001, statistical significance from Aβ1–42-stimulated levels.
Figure 7.
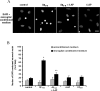
Effects of 4-AP on Aβ1–42-induced COX-2-expressing microglia. A, Representative photomicrographs of COX-2-stained microglia. Green and blue indicate staining for COX-2- and DAPI-positive nuclei, respectively. Under control conditions, little or no COX-2 expression was evident. Treatment of microglia for 24 h with Aβ1–42 (5 μ
m
) induced an intense expression of COX-2. Aβ1–42 in the presence of 4-AP (2 m
m
) treatment inhibited production of COX-2. 4-AP alone had no effect on basal levels of COX-2 production. B, The percentage of COX-2-positive microglia relative to total cells is shown under the different experimental conditions. Data are means ± SEM from six independent experiments. *p < 0.001, significance compared with control; and **p < 0.01, significance compared with Aβ1–42.
Figure 8.
Effects of microglial conditioned medium on neuronal survival. A, Representative photomicrographs of DAPI-stained primary hippocampal neurons treated for 16 h with microglial conditioned medium [microglia stimulated for 48 h with Aβ1–42 (5 μ
m
), 4-AP (2 m
m
), each alone, or in combination]. Condensed (arrow) and fragmented (arrowhead) nuclei indicate damaged neurons. Scale bar, 20 μm. B, Summary of microglial-mediated neurotoxicity results from n = 5 independent experiments and corresponding control experiments (neurons treated with unconditioned medium) from n = 5 independent experiments. *p < 0.001, statistically significant from medium of unstimulated microglia; **p < 0.001, statistically significant from conditioned medium of Aβ1–42-stimulated microglia.
Figure 9.
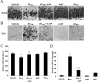
Effects of 4-AP on Aβ1–42-induced hippocampal neuron degeneration and microglial activation in vivo. A, Representative photographs of tissue sections stained with NeuN antibody from the superior blade of dentate granule cell layer taken 7 d after injection with vehicle, Aβ1–42 (1 nmol), Aβ1–42 plus 4-AP (1 mg/kg, i.p.), and 4-AP and Aβ42–1 (1 nmol). B, Representative photographs of tissue sections stained with ED1 from the superior blade of dentate granule cell layer taken from vehicle-injected rats, Aβ1–42 (1 nmol), Aβ1–42 plus 4-AP (1 mg/kg), and 4-AP or Aβ42–1 (1 nmol) at 7 d after injection. C, Quantification of the effects of Aβ1–42, 4-AP, and Aβ1–42 in the presence of 4-AP on NeuN-positive neurons. Data are mean ± SEM (n = 4/group). *p < 0.05 versus vehicle; **p < 0.05 versus Aβ1–42. D, Quantification of the effects of Aβ1–42, 4-AP, and Aβ1–42 in the presence of 4-AP on ED1-positive microglia. Data are mean ± SEM (n = 4/group). *p < 0.05 versus vehicle; **p < 0.05 versus Aβ1–42. Scale bars, 50 μm.
Similar articles
- Nuclear factor-kappaB activation by reactive oxygen species mediates voltage-gated K+ current enhancement by neurotoxic beta-amyloid peptides in nerve growth factor-differentiated PC-12 cells and hippocampal neurones.
Pannaccione A, Secondo A, Scorziello A, Calì G, Taglialatela M, Annunziato L. Pannaccione A, et al. J Neurochem. 2005 Aug;94(3):572-86. doi: 10.1111/j.1471-4159.2005.03075.x. Epub 2005 Jun 22. J Neurochem. 2005. PMID: 15969743 - PACAP inhibits delayed rectifier potassium current via a cAMP/PKA transduction pathway: evidence for the involvement of I k in the anti-apoptotic action of PACAP.
Mei YA, Vaudry D, Basille M, Castel H, Fournier A, Vaudry H, Gonzalez BJ. Mei YA, et al. Eur J Neurosci. 2004 Mar;19(6):1446-58. doi: 10.1111/j.1460-9568.2004.03227.x. Eur J Neurosci. 2004. PMID: 15066141 - The mixed-lineage kinase 3 inhibitor URMC-099 facilitates microglial amyloid-β degradation.
Dong W, Embury CM, Lu Y, Whitmire SM, Dyavarshetty B, Gelbard HA, Gendelman HE, Kiyota T. Dong W, et al. J Neuroinflammation. 2016 Jul 11;13(1):184. doi: 10.1186/s12974-016-0646-z. J Neuroinflammation. 2016. PMID: 27401058 Free PMC article. - Cellular mechanisms for amyloid beta-protein activation of rat cholinergic basal forebrain neurons.
Jhamandas JH, Cho C, Jassar B, Harris K, MacTavish D, Easaw J. Jhamandas JH, et al. J Neurophysiol. 2001 Sep;86(3):1312-20. doi: 10.1152/jn.2001.86.3.1312. J Neurophysiol. 2001. PMID: 11535679 - Androgen alleviates neurotoxicity of β-amyloid peptide (Aβ) by promoting microglial clearance of Aβ and inhibiting microglial inflammatory response to Aβ.
Yao PL, Zhuo S, Mei H, Chen XF, Li N, Zhu TF, Chen ST, Wang JM, Hou RX, Le YY. Yao PL, et al. CNS Neurosci Ther. 2017 Nov;23(11):855-865. doi: 10.1111/cns.12757. Epub 2017 Sep 20. CNS Neurosci Ther. 2017. PMID: 28941188 Free PMC article.
Cited by
- Status epilepticus induces a particular microglial activation state characterized by enhanced purinergic signaling.
Avignone E, Ulmann L, Levavasseur F, Rassendren F, Audinat E. Avignone E, et al. J Neurosci. 2008 Sep 10;28(37):9133-44. doi: 10.1523/JNEUROSCI.1820-08.2008. J Neurosci. 2008. PMID: 18784294 Free PMC article. - Microglial VEGF receptor response is an integral chemotactic component in Alzheimer's disease pathology.
Ryu JK, Cho T, Choi HB, Wang YT, McLarnon JG. Ryu JK, et al. J Neurosci. 2009 Jan 7;29(1):3-13. doi: 10.1523/JNEUROSCI.2888-08.2009. J Neurosci. 2009. PMID: 19129379 Free PMC article. - Roles of Microglial Ion Channel in Neurodegenerative Diseases.
Cojocaru A, Burada E, Bălșeanu AT, Deftu AF, Cătălin B, Popa-Wagner A, Osiac E. Cojocaru A, et al. J Clin Med. 2021 Mar 17;10(6):1239. doi: 10.3390/jcm10061239. J Clin Med. 2021. PMID: 33802786 Free PMC article. Review. - Mechanisms by which fibroblast growth factor 20 improves motor performance in a mouse model of Parkinson's disease.
Wang AQ, Kong LN, Meng MZ, Zhao XH, Chen S, Wang XT. Wang AQ, et al. Neural Regen Res. 2019 Aug;14(8):1438-1444. doi: 10.4103/1673-5374.253527. Neural Regen Res. 2019. PMID: 30964070 Free PMC article. - A leaky blood-brain barrier, fibrinogen infiltration and microglial reactivity in inflamed Alzheimer's disease brain.
Ryu JK, McLarnon JG. Ryu JK, et al. J Cell Mol Med. 2009 Sep;13(9A):2911-25. doi: 10.1111/j.1582-4934.2008.00434.x. Epub 2008 Jul 24. J Cell Mol Med. 2009. PMID: 18657226 Free PMC article.
References
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, MacKenzie IR, McGeer PL, et al. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000;21:383–421. - PMC - PubMed
- Arends YM, Duyckaerts C, Rozemuller JM, Eikelenboom P, Hauw JJ. Microglia, amyloid and dementia in Alzheimer disease. A correlative study. Neurobiol Aging. 2000;21:39–47. - PubMed
- Boland K, Behrens M, Choi D, Manias K, Perlmutter DH. The serpin-enzyme complex receptor recognizes soluble, nontoxic amyloid-beta peptide but not aggregated, cytotoxic amyloid-beta peptide. J Biol Chem. 1996;271:18032–18044. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous