Early and rapid engraftment of bone marrow-derived microglia in scrapie - PubMed (original) (raw)
Comparative Study
Early and rapid engraftment of bone marrow-derived microglia in scrapie
Josef Priller et al. J Neurosci. 2006.
Abstract
Prion neuroinvasion is accompanied by maximal activation of microglia, the significance of which for pathogenesis is unknown. Here, we used bone marrow (BM) cells expressing GFP (green fluorescent protein) to study the turnover of microglia in mouse scrapie. We found that >or=50% of all brain microglia were replaced by BM-derived cells before clinical disease onset. In terminally sick mice, microglia density increased threefold to fourfold. Hence BM-derived microglia rapidly and efficaciously colonize the brain in scrapie. Whereas reconstitution of wild-type mice with prion protein-deficient (Prnp(o/o)) BM did not alter scrapie pathogenesis, Prnp(o/o) mice transplanted with wild-type BM cells were resistant to peripherally administered prions despite high levels of infectivity in the spleen. Cerebellar homogenates from prion-inoculated Prnp(o/o) mice reconstituted with >10% of wild-type microglia failed to infect transgenic mice overexpressing the cellular prion protein. Hence, in contrast to previous reports, microglia are not competent for efficient prion transport and replication in vivo.
Figures
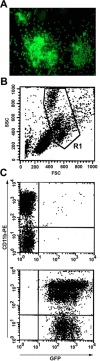
Figure 1.
Reconstitution of hematopoiesis with GFP-marked peripheral blood cell progeny. A, BMcells were transduced with a murine stem cell virus-based retroviral vector encoding GFP. Cells were subsequently plated in methylcellulose supplemented with hematopoietic growth factors. After 6 d in culture, GFP-transduced hematopoietic colonies were visualized under fluorescence microscopy.B, Fifteen weeks after transplantation of GFP-transducedBMcells into lethally irradiated mice, myeloid cells were gated based on their forward-scatter (FSC) and side-scatter (SSC) profile.C, Flow cytometry of GFP expression in peripheral blood mononuclear cells revealed that>99% of CD11b+ cells expressed GFP in a representative wt→wt chimera (bottom). For comparison, no GFP expression was detected in peripheral blood mononuclear cells from a nontransplanted wt mouse (top).
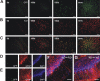
Figure 2.
Engraftment of BM-derived microglia after intraperitoneal prion inoculation. Wild-type mice transplanted with GFP-transduced BM cells were inoculated intraperitoneally with scrapie prions at 15 weeks post-BMT. A, Representative cerebellar sections of chimeric mice reveal engraftment of GFP+ BM-derived cells at the time of prion inoculation (0d), 100 and 150 dpi, and in terminal stages of scrapie (term.). B, Immunohistochemical staining of the same sections for the monocyte/macrophage marker, Iba1 (red), reveals enhanced engraftment of BM-derived microglia at 150 d, and microglia proliferation in terminally ill mice (C, overlay of images in A and B). D, Representative cerebellar section of a ko mouse transplanted with GFP-transduced wt BM cells (wt→ko) at 160 dpi. E, Representative cerebellar section of a terminally ill wt mouse transplanted with GFP-transduced ko BM cells (ko→wt) reveals engraftment of GFP+ BM-derived microglia and substantial astrogliosis (red, GFAP immunoreactivity; blue, DAPI staining of nuclei). G, Overlay of the images in E. Scale bars: A–C, 100 μm; F, G, 25 μm.
Figure 3.
Semiquantitative analysis of regional microglia engraftment at various times after intraperitoneal prion inoculation. The numbers of Iba1+ microglia (white bars) and GFP+Iba1+ BM-derived microglia (gray bars) were determined in representative sections from brainstem (A), cerebellum (B), hippocampus (C), and frontal cortex (D) of chimeric mice at 0–300 dpi and in terminal stages of scrapie (term.). Data are expressed as microglial cells per square millimeter (means + SD). Note the significant increase in BM-derived microglia at 150 dpi in wt→wt compared with wt→ko and ko→ko mice (p < 0.05). Moreover, the number of microglia, including BM-derived microglia, was significantly increased in terminal (term.) wt→wt compared with wt→wt mice at 150 dpi (p < 0.05) and wt→ko and ko→ko mice at 300 dpi (p < 0.05). The number of cerebellar microglia was significantly decreased in wt→ko and ko→ko mice at 300 dpi compared with 150 dpi (p < 0.01).
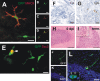
Figure 4.
Characterization of BM-derived cells in the scrapie-affected brain. Representative images taken from the cerebellum of a terminally ill ko→wt mouse. A, A GFP-expressing microglial cell is immunoreactive for MHC class II (red), whereas a neighboring GFP+ cell does not express MHCII (overlay of GFP and Texas Red fluorescence). B, Single optical section through GFP-expressing microglia, which show MHCII immunoreactivity (C). D, The overlay of the confocal microscopic images in B and C reveals coexpression of GFP and MHCII. E, A proliferating GFP-expressing microglia shows nuclear Tec3 immunoreactivity (red). Neighboring BM-derived microglia do not express the proliferation marker (overlay of GFP and Texas Red fluorescence). F, G, Immunohistochemical staining for IgG visualized by diaminobenzidine, and counterstaining with hematoxylin reveals the absence of IgG extravasation into the neuropil (negative control is shown in F). H, I, Hematoxylin and eosin stainings of cerebellar sections from wt→wt mice at 0 dpi (H) and terminal disease (I). Note the spongiform encephalopathy in I (a higher magnification is shown as an inset). J, Single optical section through a GFP-expressing Purkinje cell, which is surrounded by GFP+ microglia. K, DAPI staining of the nuclei. L, The overlay of the confocal microscopic images in J and K reveals that the GFP-expressing Purkinje cell contains two nuclei (indicated by arrowheads), which is suggestive of cell fusion. A higher magnification of the perikaryon is shown as an inset. Scale bars: A–D, 10 μm; E, 5 μm; F, G, 20 μm; H, I, 100 μm; (in L) J–L, 50 μm.
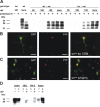
Figure 5.
Expression of PrP in BM-derived microglia. A, Immunoblot analysis of brain homogenates electrophoresed in the native state (−) or after digestion with proteinase K (PK; +). Large amounts of PK-resistant PrPSc were detected in the brains of all mice that had developed scrapie (term.): wt mice, and wt→wt and ko→wt chimeras. No PrPSc accumulation was detected in clinically healthy ko mice at 347 dpi, and in wt→wt and ko→wt chimeras at 50 and 100 dpi. Subclinical scrapie was evident in wt→wt and ko→wt mice at 150 dpi. Note that the amount of PrPSc was lower in ko→wt compared with wt→wt mice at 150 dpi. B, A GFP-expressing microglial cell (left) is immunoreactive for PrP (middle; Alexa staining) in a wt→ko chimera at 150 dpi (right; overlay of the images). C, In a wt→wt mouse that had developed scrapie, activated GFP-expressing microglia and macrophages (left) show PrP immunoreactivity (middle; Alexa staining). Overlay of the images (right) reveals that BM-derived cells contain most of the PrP immunoreactivity. D, Immunoblot analysis of brain homogenates and lysates of primary microglial cultures (MG), BV-2 cells and primary astroglial cultures (Astro). Expression of PrP was comparatively low in microglia and BV-2 cells, requiring immunoaffinity purification (IAP) for detection. Scale bars: B, C, 10 μm.
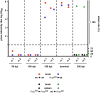
Figure 6.
Prion infectivity titers in brains and spleens of chimeric mice after intraperitoneal prion inoculation. Tg_a_20 indicator mice were inoculated intracerebrally with 1% (w/v) cerebellum or spleen homogenates from BM-chimeric mice (n = 2 per genotype and day postinoculation). Titers were determined in cerebellum from wt→wt (red circles), ko→wt (blue circles), ko→ko (black circles), and wt→ko mice (green circles) at 50, 100, 150, 300 dpi and terminal disease. Cerebellum infectivity, leading to an attack rate of 100%, was only detected in ko→wt mice at 150 dpi (4.2–5.0 log LD50/ml), wt→wt mice at 150 dpi (5.1–5.7 log LD50/ml), terminal ko→wt mice (5.4 log LD50/ml), and terminal wt→wt mice (5.2–5.7 log LD50/ml). Titers were also determined in spleens from ko→ko (black triangles) and wt→ko mice (green triangles) at 300 dpi. Spleen infectivity was only detected in wt→ko mice (5.4 log LD50/ml).
Similar articles
- Fewer PrPc myeloid-based cells in sheep with the prion-resistant genotype.
Herrmann LM, Baszler TV, O'Rourke KI, Suarez CE, Bakko M, Alverson J, Knowles DP. Herrmann LM, et al. Neuroreport. 2006 Feb 6;17(2):125-9. doi: 10.1097/01.wnr.0000198430.39691.3c. Neuroreport. 2006. PMID: 16407757 - PrP-expressing tissue required for transfer of scrapie infectivity from spleen to brain.
Blättler T, Brandner S, Raeber AJ, Klein MA, Voigtländer T, Weissmann C, Aguzzi A. Blättler T, et al. Nature. 1997 Sep 4;389(6646):69-73. doi: 10.1038/37981. Nature. 1997. PMID: 9288968 - Analysis of protein levels of 24 cytokines in scrapie agent-infected brain and glial cell cultures from mice differing in prion protein expression levels.
Tribouillard-Tanvier D, Striebel JF, Peterson KE, Chesebro B. Tribouillard-Tanvier D, et al. J Virol. 2009 Nov;83(21):11244-53. doi: 10.1128/JVI.01413-09. Epub 2009 Aug 26. J Virol. 2009. PMID: 19710140 Free PMC article. - Prions: pathogenesis and reverse genetics.
Aguzzi A, Klein MA, Montrasio F, Pekarik V, Brandner S, Furukawa H, Käser P, Röckl C, Glatzel M. Aguzzi A, et al. Ann N Y Acad Sci. 2000;920:140-57. doi: 10.1111/j.1749-6632.2000.tb06916.x. Ann N Y Acad Sci. 2000. PMID: 11193143 Review. - [Mechanisms of neuroinvasion by prions: molecular principles and present state of research].
Brandner S, Klein MA, Aguzzi A. Brandner S, et al. Schweiz Med Wochenschr. 2000 Mar 25;130(12):435-42. Schweiz Med Wochenschr. 2000. PMID: 10780058 Review. German.
Cited by
- Is Alzheimer disease a failure of mobilizing immune defense? Lessons from cognitively fit oldest-old.
Katsel P, Haroutunian V. Katsel P, et al. Dialogues Clin Neurosci. 2019 Mar;21(1):7-19. doi: 10.31887/DCNS.2019.21.1/vharoutunian. Dialogues Clin Neurosci. 2019. PMID: 31607776 Free PMC article. - Deposition pattern and subcellular distribution of disease-associated prion protein in cerebellar organotypic slice cultures infected with scrapie.
Wolf H, Hossinger A, Fehlinger A, Büttner S, Sim V, McKenzie D, Vorberg IM. Wolf H, et al. Front Neurosci. 2015 Nov 4;9:410. doi: 10.3389/fnins.2015.00410. eCollection 2015. Front Neurosci. 2015. PMID: 26581229 Free PMC article. - Indications for cellular migration from the central nervous system to its draining lymph nodes in CD11c-GFP+ bone-marrow chimeras following EAE.
Schiefenhövel F, Immig K, Prodinger C, Bechmann I. Schiefenhövel F, et al. Exp Brain Res. 2017 Jul;235(7):2151-2166. doi: 10.1007/s00221-017-4956-x. Epub 2017 Apr 18. Exp Brain Res. 2017. PMID: 28421248 - Impacts of systemic milieu on cerebrovascular and brain aging: insights from heterochronic parabiosis, blood exchange, and plasma transfer experiments.
Gulej R, Patai R, Ungvari A, Kallai A, Tarantini S, Yabluchanskiy A, Huffman DM, Conboy MJ, Conboy IM, Kivimäki M, Csiszar A, Ungvari Z. Gulej R, et al. Geroscience. 2025 May 23. doi: 10.1007/s11357-025-01657-y. Online ahead of print. Geroscience. 2025. PMID: 40407975 Review. - Origin of microglia: current concepts and past controversies.
Ginhoux F, Prinz M. Ginhoux F, et al. Cold Spring Harb Perspect Biol. 2015 Jul 1;7(8):a020537. doi: 10.1101/cshperspect.a020537. Cold Spring Harb Perspect Biol. 2015. PMID: 26134003 Free PMC article. Review.
References
- Aguzzi A, Heikenwalder M. Prions, cytokines, and chemokines: a meeting in lymphoid organs. Immunity. 2005;22:145–154. - PubMed
- Aguzzi A, Polymenidou M. Mammalian prion biology: one century of evolving concepts. Cell. 2004;116:313–327. - PubMed
- Aguzzi A, Sigurdson CJ. Antiprion immunotherapy: to suppress or to stimulate? Nat Rev Immunol. 2004;4:725–736. - PubMed
- Andréoletti O, Berthon P, Levavasseur E, Marc D, Lantier F, Monks E, Elsen JM, Schelcher F. Phenotyping of protein-prion (PrPsc)-accumulating cells in lymphoid and neural tissues of naturally scrapie-affected sheep by double-labeling immunohistochemistry. J Histochem Cytochem. 2002a;50:1357–1370. - PubMed
- Andréoletti O, Levavasseur E, Uro-Coste E, Tabouret G, Sarradin P, Delisle MB, Berthon P, Salvayre R, Schelcher F, Negre-Salvayre A. Astrocytes accumulate 4-hydroxynonenal adducts in murine scrapie and human Creutzfeldt-Jakob disease. Neurobiol Dis. 2002b;11:386–393. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical