The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized - PubMed (original) (raw)
doi: 10.1016/j.ccr.2006.08.027.
Andrew H Wei, Kylie D Mason, Cassandra J Vandenberg, Lin Chen, Peter E Czabotar, Simon N Willis, Clare L Scott, Catherine L Day, Suzanne Cory, Jerry M Adams, Andrew W Roberts, David C S Huang
Affiliations
- PMID: 17097561
- PMCID: PMC2953559
- DOI: 10.1016/j.ccr.2006.08.027
The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized
Mark F van Delft et al. Cancer Cell. 2006 Nov.
Abstract
Since apoptosis is impaired in malignant cells overexpressing prosurvival Bcl-2 proteins, drugs mimicking their natural antagonists, BH3-only proteins, might overcome chemoresistance. Of seven putative BH3 mimetics tested, only ABT-737 triggered Bax/Bak-mediated apoptosis. Despite its high affinity for Bcl-2, Bcl-x(L), and Bcl-w, many cell types proved refractory to ABT-737. We show that this resistance reflects ABT-737's inability to target another prosurvival relative, Mcl-1. Downregulation of Mcl-1 by several strategies conferred sensitivity to ABT-737. Furthermore, enforced Mcl-1 expression in a mouse lymphoma model conferred resistance. In contrast, cells overexpressing Bcl-2 remained highly sensitive to ABT-737. Hence, ABT-737 should prove efficacious in tumors with low Mcl-1 levels, or when combined with agents that inactivate Mcl-1, even to treat those tumors that overexpress Bcl-2.
Figures
Figure 1
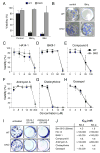
Many putative BH3 mimetics do not kill like BH3-only proteins A: The viability of wild-type MEFs (WT) or Bax- and Bak-deficient MEFs (DKO) 24 h after infection with the indicated retroviruses. Expression of the cDNA encoding the BH3-only protein BimS or tBid was linked by an IRES to that of GFP, and the viability of GFP+ve cells determined by PI exclusion. B: Representative wells showing colony formation by wild-type (WT) or Bax/Bak-deficient (DKO) MEFs after infection with the control parental retrovirus or one expressing BimL. C–H: The viability (% cells excluding PI) of WT or Bax- and Bak-deficient (DKO) MEFs treated for 24 h with graded doses of the indicated putative BH3 mimetics. I: Colonies formed by wild-type (WT) or Bax/Bak-deficient (DKO) MEFs in the presence of no treatment, HA14-1 or Antimycin A. J: The relative affinities (IC50 in nM) of a BimBH3 peptide (as previously reported; Chen et al., 2005) and several putative BH3 mimetic compounds for Bcl-2 and/or Bcl-w. The affinities were measured in solution competition assays (Chen et al., 2005). Data in A and C–H represent means ± SD from 3 independent experiments.
Figure 2
ABT-737 cooperates with Noxa to induce Bax/Bak-dependent killing A: The viability of wild-type MEFs (WT), Bax/Bak-deficient MEFs (DKO), and Bak- or Bax-singly deficient MEFs was determined by PI exclusion 48 h after exposure to ABT-737 (10 μM) or Etoposide (10 μM). B: ABT-737 is a Bad BH3 mimetic. Based on the relative affinities (IC50 in nM) of ABT-737 for mammalian pro-survival proteins, determined in solution competition assays (Fig. S1A), ABT-737 and Bad bind to the same subset of Bcl-2 pro-survival proteins. According to our model for initiating the apoptotic program (Chen et al., 2005; Willis et al., 2005), Bad and Noxa are poor inducers of apoptosis individually because each binds only a subset of the pro-survival proteins, whereas Bim is a potent killer because it binds all of them. By this rationale, ABT-737 (like Bad) should also cooperate with Noxa to kill cells. C: Noxa triggers Mcl-1 degradation. Immunoblots of lysates prepared from the MEFs after retroviral infection with wild-type Noxa or the 3E mutant (an inactive mutant that does not bind Mcl-1) probed for Mcl-1 and HSP70 (loading control). D: Noxa sensitizes wild-type MEFs to ABT-737 killing. Wild-type MEFs expressing wild-type human Noxa or an inactive mutant (Noxa 3E) (Willis et al., 2005), were exposed to ABT-737 for 8 h and their viability determined. E: Bax/Bak-deficient MEFs (DKO) are resistant to ABT-737 even when Mcl-1 is targeted. Long-term clonogenic survival of cells exposed to ABT-737. Equal numbers of the indicated MEFs, or their counterparts stably expressing Noxa or the inactive Noxa 3E, were plated in media containing vehicle or ABT-737 (1 μM, replenished after 3 d) and the colonies formed scored after 6 d. The number of colonies obtained with ABT-737 treatment is expressed as a proportion of colonies formed with the vehicle alone. - no colonies. F: Either Bax or Bak can mediate killing by ABT-737 provided Mcl-1 is targeted. Viability of the indicated MEFs stably expressing Noxa was determined 8 h after exposure to ABT-737. Note that Bax/Bak-deficient MEFs (DKO) are resistant. G: Noxa sensitizes FDC-P1 myeloid cells to ABT-737 killing. The viabilities of FDC-P1 cells, retrovirally infected to express Noxa, mutant Noxa 3E or Bad, were compared after a 24 h treatment with graded doses of ABT-737. Data in A and D–G represent means ± SD from a representative of 3 experiments.
Figure 3
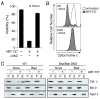
ABT-737 induces cytochrome c release and caspase-dependent apoptosis when Mcl-1 is neutralized A: Cell death triggered by ABT-737 is caspase dependent. Noxa-expressing wild-type MEFs were treated with ABT-737 (1 μM) and their viability was assessed by PI exclusion; co-incubation with the broad-spectrum caspase inhibitor zVAD. fmk (50 μM) abrogated ABT-737 killing at this time point. Data represent means ± SD from a representative of 3 experiments. B: ABT-737 induces cytochrome c release when Mcl-1 is neutralized. Noxa-expressing wild-type (WT) or Bax/Bak-deficient MEFs (DKO) were exposed to ABT-737 (10 μM for 4 h), permeabilized with digitonin to wash out any cytochrome c released to the cytosol and then fixed. Residual mitochondrial cytochrome c was detected by immunostaining and flow cytometry (Waterhouse et al., 2004). ABT-737 triggered loss of cytochrome c from the mitochondria of WT MEFs, as indicated by the peak of weaker staining (compare filled with unfilled histogram; upper), but not from the Bax/Bak-deficient DKO MEFs (lower). C: ABT-737 and Noxa cooperate in vitro to release cytochrome c. Lysates prepared from wild-type (left) or Bax/Bak-deficient MEFs (DKO; right) stably expressing Noxa or Bad were incubated with vehicle (−) or 5 μM ABT-737 (+), before fractionation into the pellet (P) and supernatant (S) fractions. Equivalent fractions were probed for cytochrome c, Bcl-2 (membrane fraction marker) and Apaf-1 (cytosolic marker).
Figure 4
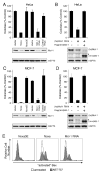
Neutralizing Mcl-1 sensitizes different cell types to ABT-737 Colony formation after continuous exposure to ABT-737 (1 μM, replenished every 3 d) of HeLa (A, B) or MCF-7 cells (C, D) infected with empty vectors, or stably expressing Noxa, mutant Noxa 3E, RNAi targeting Mcl-1, or RNAi to an irrelevant target (control RNAi). Introduction of mouse Mcl-1, which is not targeted by the human specific Mcl-1 RNAi construct, restored the resistance in HeLa (B) or MCF-7 cells (D) to ABT-737. Clonogenic survival data (after 7 d) are representative means ± SD of 3 independent experiments. (A, B) The lower panels are immunoblots for Mcl-1 or HSP70 (loading control). (C, D) The lower panels are immunoblots for human Mcl-1 (top), mouse Mcl-1 (middle: * residual signal from human Mcl-1 probe) or HSP70 (lower panel). E: ABT-737 triggers Bax activation when Mcl-1 is neutralized. HeLa cells expressing mutant Noxa 3E, Noxa or Mcl-1 RNAi, were treated for 4 h with ABT-737 (10 μM), and Bax activation detected by flow cytometric analysis after staining permeabilized cells with an antibody (clone 3) that specifically recognizes activated Bax (Willis et al., 2005).
Figure 5
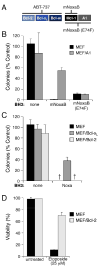
Pro-survival proteins differ in their ability to antagonize ABT-737 A: Noxa variants that selectively neutralize Mcl-1 or both Mcl-1 and A1. Whereas the human Noxa used in Figs. 2–4 (above) binds both Mcl-1 and A1 (Chen et al., 2005) (Fig. 2), the mouse Noxa BH3 B region (mNoxaB) only binds tightly to Mcl-1 (IC50 60 nM; IC50 > 2μM for all other pro-survival proteins). The E74F mutant of mNoxaB binds tightly to both Mcl-1 and A1 (IC50Mcl-1 24 nM, IC50A1 12nM), but has weaker affinity (IC50 > 2 μM) for all other pro-survival proteins. The affinities were measured in solution competition assays (Chen et al., 2005). B: A1 expression confers partial resistance to ABT-737. Colony formation after 6 d by parental wild-type MEFs or MEFs stably overexpressing FLAG-tagged A1 in the presence of ABT-737 (1 μM, replenished after 3 d) and the indicated BH3 domains, placed within an otherwise inert BimS backbone lacking its own BH3 (Chen et al., 2005) and expressed from retroviruses. C, D: Killing by ABT-737 is not inhibited by Bcl-2 and only partially by Bcl-xL. Wild-type MEFs, or MEFs overexpressing FLAG-tagged Bcl-xL or Bcl-2, were tested for their sensitivity to ABT-737 (1μM) in the presence human Noxa. The Bcl-2 overexpression did not rescue any colony formation, even though it inhibited apoptosis induced by 24 h exposure to Etoposide (D). - no colonies. Data in B–D represent means ± SD from a representative of 3 experiments.
Figure 6
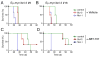
Mcl-1 expression blunts the in vivo response of Eμ-myc/_bcl_-2 bi-transgenic lymphomas to ABT-737 Two independent progenitor B cell lymphomas (#9: A, C and #16: B, D) derived from Eμ-myc/_bcl_-2 bi-transgenic mice (Strasser et al., 1990) were infected with the control GFP expressing retrovirus, or ones co-expressing Bcl-2 or Mcl-1 and GFP. The mice were injected with 106 infected tumor cells before initiating therapy 4 d later with (C, D) ABT-737 (75 mg/kg given daily for two weeks by intraperitoneal injection) or the (A, B) vehicle alone. ABT-737 improved the survival of mice transplanted with both tumors even when Bcl-2 was overexpressed. However, Mcl-1 overexpressing lymphomas were highly resistant to ABT-737 and these mice died rapidly, akin to their untreated counterparts. Kaplan-Meier survival curves were derived from an experiment with 3 mice in each cohort.
Figure 7
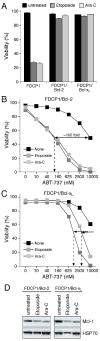
ABT-737 potently sensitizes cells overexpressing Bcl-2 to genotoxic agents A: Bcl-2 or Bcl-xL overexpression renders FDC-P1 cells resistant to genotoxic agents. FDC-P1 cells or FDC-P1 cells overexpressing Bcl-2 or Bcl-xL were treated with Etoposide (25 μM) or Cytosine Arabinoside (25 μM) for 24 h and viability determined by PI exclusion. B, C: FDC-P1 cells overexpressing Bcl-2 (B) or Bcl-xL (C) were treated with ABT-737 (0–10 μM), and Etoposide (25μM) or Cytosine Arabinoside (Ara-C; 25 μM) or no other drug (none) for 24 h and the viability determined by PI exclusion. Filled lines - fold increase in killing efficacy; hatched lines - EC50 values. D: Cytotoxic agents trigger Mcl-1 degradation. Equivalent amounts of lysates prepared from cells overexpressing Bcl-2 or Bcl-xL that were left untreated or after 24 h exposure to Etoposide (25 μM) or Ara-C (25 μM) were probed for Mcl-1 or HSP70 (loading control). Data in A–C represent means ± SD from a representative experiment.
Figure 8
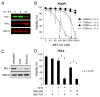
Alternative ways to target Mcl-1 and sensitize cells to ABT-737 A: IL-3 withdrawal triggers Mcl-1 degradation and Bim accumulation in FDC-P1 cells. Lysates prepared from Bcl-2-overexpressing FDC-P1 cells grown for 0–24 h in the absence of its essential growth factor IL-3 were blotted for Mcl-1, Bim or HSP70 (loading control). B: IL-3 deprivation sensitizes FDC-P1 cells overexpressing Bcl-2 (squares) or Bcl-xL (circles) to ABT-737. Viability was determined for the cells, cultured with (filled symbols) or without (unfilled symbols) IL-3 and exposed to ABT-737 (0–10 μM) for 24 h. C: The protein synthesis inhibitor cycloheximide (CHX) and the CDK inhibitor Seliciclib both reduce Mcl-1 expression. HeLa cells were treated with 50 μg/mL cycloheximide or 30 μM Seliciclib (R-roscovitine/CYC202) for 12 h and Mcl-1 expression measured by immunoblotting (HSP-70, loading control). D: HeLa cells were left untreated, treated with 2.5 μM ABT-737, 50 μg/mL cycloheximide or 30 μM Seliciclib (R-roscovitine/CYC202), or combinations of ABT-737 with cycloheximide or Seliciclib, for 14 h. Statistical analyses were performed using two-tailed unpaired Student’s t-test. Data in B and D represent means ± SD from 3 independent experiments.
Comment in
- Restoring cancer's death sentence.
Letai A. Letai A. Cancer Cell. 2006 Nov;10(5):343-5. doi: 10.1016/j.ccr.2006.10.014. Cancer Cell. 2006. PMID: 17097553
Similar articles
- Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia.
Konopleva M, Contractor R, Tsao T, Samudio I, Ruvolo PP, Kitada S, Deng X, Zhai D, Shi YX, Sneed T, Verhaegen M, Soengas M, Ruvolo VR, McQueen T, Schober WD, Watt JC, Jiffar T, Ling X, Marini FC, Harris D, Dietrich M, Estrov Z, McCubrey J, May WS, Reed JC, Andreeff M. Konopleva M, et al. Cancer Cell. 2006 Nov;10(5):375-88. doi: 10.1016/j.ccr.2006.10.006. Cancer Cell. 2006. PMID: 17097560 - Mcl-1 down-regulation potentiates ABT-737 lethality by cooperatively inducing Bak activation and Bax translocation.
Chen S, Dai Y, Harada H, Dent P, Grant S. Chen S, et al. Cancer Res. 2007 Jan 15;67(2):782-91. doi: 10.1158/0008-5472.CAN-06-3964. Cancer Res. 2007. PMID: 17234790 - MEK inhibition enhances ABT-737-induced leukemia cell apoptosis via prevention of ERK-activated MCL-1 induction and modulation of MCL-1/BIM complex.
Konopleva M, Milella M, Ruvolo P, Watts JC, Ricciardi MR, Korchin B, McQueen T, Bornmann W, Tsao T, Bergamo P, Mak DH, Chen W, McCubrey J, Tafuri A, Andreeff M. Konopleva M, et al. Leukemia. 2012 Apr;26(4):778-87. doi: 10.1038/leu.2011.287. Epub 2011 Nov 8. Leukemia. 2012. PMID: 22064351 Free PMC article. Retracted. - Targeting multiple arms of the apoptotic regulatory machinery.
Dai Y, Grant S. Dai Y, et al. Cancer Res. 2007 Apr 1;67(7):2908-11. doi: 10.1158/0008-5472.CAN-07-0082. Cancer Res. 2007. PMID: 17409392 Review. - BH3 mimetics to improve cancer therapy; mechanisms and examples.
Zhang L, Ming L, Yu J. Zhang L, et al. Drug Resist Updat. 2007 Dec;10(6):207-17. doi: 10.1016/j.drup.2007.08.002. Epub 2007 Oct 24. Drug Resist Updat. 2007. PMID: 17921043 Free PMC article. Review.
Cited by
- USP30 deubiquitylates mitochondrial Parkin substrates and restricts apoptotic cell death.
Liang JR, Martinez A, Lane JD, Mayor U, Clague MJ, Urbé S. Liang JR, et al. EMBO Rep. 2015 May;16(5):618-27. doi: 10.15252/embr.201439820. Epub 2015 Mar 4. EMBO Rep. 2015. PMID: 25739811 Free PMC article. - Ubiquitination and deubiquitination of MCL1 in cancer: deciphering chemoresistance mechanisms and providing potential therapeutic options.
Wu X, Luo Q, Liu Z. Wu X, et al. Cell Death Dis. 2020 Jul 22;11(7):556. doi: 10.1038/s41419-020-02760-y. Cell Death Dis. 2020. PMID: 32699213 Free PMC article. Review. - Use of ratiometrically designed nanocarrier targeting CDK4/6 and autophagy pathways for effective pancreatic cancer treatment.
Ji Y, Liu X, Li J, Xie X, Huang M, Jiang J, Liao YP, Donahue T, Meng H. Ji Y, et al. Nat Commun. 2020 Aug 25;11(1):4249. doi: 10.1038/s41467-020-17996-7. Nat Commun. 2020. PMID: 32843618 Free PMC article. - Combination of PKCδ Inhibition with Conventional TKI Treatment to Target CML Models.
Muselli F, Mourgues L, Morcos R, Rochet N, Nebout M, Guerci-Bresler A, Faller DV, William RM, Mhaidly R, Verhoeyen E, Legros L, Peyron JF, Mary D. Muselli F, et al. Cancers (Basel). 2021 Apr 2;13(7):1693. doi: 10.3390/cancers13071693. Cancers (Basel). 2021. PMID: 33918475 Free PMC article. - Potent and selective small-molecule MCL-1 inhibitors demonstrate on-target cancer cell killing activity as single agents and in combination with ABT-263 (navitoclax).
Leverson JD, Zhang H, Chen J, Tahir SK, Phillips DC, Xue J, Nimmer P, Jin S, Smith M, Xiao Y, Kovar P, Tanaka A, Bruncko M, Sheppard GS, Wang L, Gierke S, Kategaya L, Anderson DJ, Wong C, Eastham-Anderson J, Ludlam MJ, Sampath D, Fairbrother WJ, Wertz I, Rosenberg SH, Tse C, Elmore SW, Souers AJ. Leverson JD, et al. Cell Death Dis. 2015 Jan 15;6(1):e1590. doi: 10.1038/cddis.2014.561. Cell Death Dis. 2015. PMID: 25590800 Free PMC article.
References
- Baell JB, Huang DCS. Prospects for targeting the Bcl-2 family of proteins to develop novel cytotoxic drugs. Biochem Pharmacol. 2002;64:851–863. - PubMed
- Brummelkamp T, Bernards R, Agami R. Stable suppression of tumorigenicity by virus-mediated RNA interference. Cancer Cell. 2002;2:243–247. - PubMed
- Chan SL, Lee MC, Tan KO, Yang LK, Lee AS, Flotow H, Fu NY, Butler MS, Soejarto DD, Buss AD, Yu VC. Identification of chelerythrine as an inhibitor of Bcl-xL function. J Biol Chem. 2003;278:20453–20456. - PubMed
- Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, Colman PM, Day CL, Adams JM, Huang DCS. Differential targeting of pro-survival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. - PubMed
- Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, Korsmeyer SJ. Bcl-2, Bcl-xL sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell. 2001;8:705–711. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA043540/CA/NCI NIH HHS/United States
- CA80188/CA/NCI NIH HHS/United States
- CA43540/CA/NCI NIH HHS/United States
- R01 CA043540-21/CA/NCI NIH HHS/United States
- R01 CA080188-08/CA/NCI NIH HHS/United States
- R01 CA080188/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials