Glycogen synthase kinase-3 (GSK3) in psychiatric diseases and therapeutic interventions - PubMed (original) (raw)
Review
Glycogen synthase kinase-3 (GSK3) in psychiatric diseases and therapeutic interventions
Richard S Jope et al. Curr Drug Targets. 2006 Nov.
Abstract
Glycogen synthase kinase-3 (GSK3) has recently been linked to mood disorders and schizophrenia, and the neurotransmitter systems and therapeutic treatments associated with these diseases. GSK3 is a widely influential enzyme that is capable of phosphorylating, and thereby regulating, over forty known substrates. Four mechanisms regulating GSK3 (phosphorylation, protein complexes, localization, and substrate phosphorylation) combine to provide substrate-specific regulation of the actions of GSK3. Several intracellular signaling cascades converge on GSK3 to modulate its activity, and several neurotransmitter systems also regulate GSK3, including serotonergic, dopaminergic, cholinergic, and glutamatergic systems. Because of changes in these neurotransmitter systems and the actions of therapeutic drugs, GSK3 has been linked to the mood disorders, bipolar disorder and depression, and to schizophrenia. Inhibition of GSK3 may be an important therapeutic target of mood stabilizers, and regulation of GSK3 may be involved in the therapeutic effects of other drugs used in psychiatry. Dysregulated GSK3 in bipolar disorder, depression, and schizophrenia could have multiple effects that could impair neural plasticity, such as modulation of neuronal architecture, neurogenesis, gene expression, and the ability of neurons to respond to stressful, potentially lethal, conditions. In part because of these key actions of GSK3 and its associations with mood disorders and schizophrenia, much research is currently being devoted to identifying new selective inhibitors of GSK3.
Figures
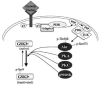
Fig 1. Regulation of the inhibitory serine-phosphorylation of GSK3
Phosphorylation of Ser-9 of GSK3β inhibits its activity. Some of the kinases reported to phosphorylate this site on GSK3β include Akt, protein kinase A (PKA; also known as cyclic AMP-dependent protein kinase), protein kinase C (PKC), and p90 ribosomal S6 kinase (p90RSK). Signaling leading from a growth factor receptor to activation of Akt is depicted. Growth factor receptor stimulation causes tyrosine phosphorylation (pY) of the receptor which interacts with various adaptor proteins to initiate a signaling cascade that results in activation of Akt via dual phosphorylation on threonine-308 and serine-473 which results in Akt-induced serine-phosphorylation and inactivation of GSK3β . ILK, integrin-linked kinase; PDK, phosphoinositide-dependent kinase; PI3K, phosphoinositide 3-kinase; PIP2, phosphatidylinositol-4,5-bisphosphate; PIP3, phosphatidylinositol-3,4,5-trisphosphate.
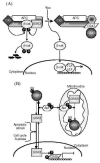
Fig 2. Mechanisms contributing to substrate-selective regulation of GSK3
A. GSK3-binding proteins. GSK3-binding proteins regulate the action of GSK3 in the Wnt signaling pathway. Axin acts as a scaffold bringing together the substrate β -catenin with APC, the priming kinase, casein kinase 1 (CK1), and GSK3. In the absence of Wnt, phosphorylation of β -catenin by CK1 and GSK3 targets it for degradation. Wnt activation results in disheveled (dvl) and FRAT binding to GSK3, inhibiting its phosphorylation of β -catenin. This results in the stabilization and accumulation of β -catenin, its translocation to the nucleus, and facilitation of TCF/LEF-mediated transcription. APC, adenomatous polyposis coli gene product; FRAT, frequently rearranged in advanced T-cell lymphoma; LEF, lymphoid-enhancing factor; TCF, T cell factor. B. Subcellular distribution. GSK3 is predominantly a cytosolic protein, where it can be regulated by serine phosphorylation carried out by several kinases, such as Akt. GSK3 reversibly translocates to the nucleus where it’s level is increased in the S-phase of the cell cycle and during some types of apoptosis. Nuclear accumulation of GSK3 facilitates its actions on nuclear substrates, such as the transcription factor cyclic AMP response element-binding protein (CREB), which is inhibited following phosphorylation by GSK3. Mitochondria also contain GSK3. Although no studies have reported alterations of mitochondrial GSK3 levels, its activity can be regulated by serine phosphorylation. For example, activation of cytosolic Akt can lead to its import into mitochondria where it can serine-phosphorylate mitochondrial GSK3 to inhibit its activity. C. Priming phosphorylation of GSK3 substrates. Many substrates of GSK3 must be "primed", which means they are pre-phosphorylated at a serine/threonine four residues removed from the serine/threonine that is phosphorylated by GSK3. Thus, the consensus site for phosphorylation of primed substrates by GSK3 is S/T-X-X-X-S/T(p). This provides an important regulatory mechanism controlling the action of GSK3 because signaling pathways phosphorylating its substrates must be active before GSK3 can have any effect on such substrates. The preference of GSK3 for phosphorylating substrates that have been prephosphorylated (or "primed") 4 amino acids C-terminal to the target Ser/Thr is due to the presence of a phosphate binding pocket in GSK3. The phosphate of the primed substrate sits in this pocket and positions the phosphate acceptor site to enable efficient phosphorylation by GSK3.
Fig 2. Mechanisms contributing to substrate-selective regulation of GSK3
A. GSK3-binding proteins. GSK3-binding proteins regulate the action of GSK3 in the Wnt signaling pathway. Axin acts as a scaffold bringing together the substrate β -catenin with APC, the priming kinase, casein kinase 1 (CK1), and GSK3. In the absence of Wnt, phosphorylation of β -catenin by CK1 and GSK3 targets it for degradation. Wnt activation results in disheveled (dvl) and FRAT binding to GSK3, inhibiting its phosphorylation of β -catenin. This results in the stabilization and accumulation of β -catenin, its translocation to the nucleus, and facilitation of TCF/LEF-mediated transcription. APC, adenomatous polyposis coli gene product; FRAT, frequently rearranged in advanced T-cell lymphoma; LEF, lymphoid-enhancing factor; TCF, T cell factor. B. Subcellular distribution. GSK3 is predominantly a cytosolic protein, where it can be regulated by serine phosphorylation carried out by several kinases, such as Akt. GSK3 reversibly translocates to the nucleus where it’s level is increased in the S-phase of the cell cycle and during some types of apoptosis. Nuclear accumulation of GSK3 facilitates its actions on nuclear substrates, such as the transcription factor cyclic AMP response element-binding protein (CREB), which is inhibited following phosphorylation by GSK3. Mitochondria also contain GSK3. Although no studies have reported alterations of mitochondrial GSK3 levels, its activity can be regulated by serine phosphorylation. For example, activation of cytosolic Akt can lead to its import into mitochondria where it can serine-phosphorylate mitochondrial GSK3 to inhibit its activity. C. Priming phosphorylation of GSK3 substrates. Many substrates of GSK3 must be "primed", which means they are pre-phosphorylated at a serine/threonine four residues removed from the serine/threonine that is phosphorylated by GSK3. Thus, the consensus site for phosphorylation of primed substrates by GSK3 is S/T-X-X-X-S/T(p). This provides an important regulatory mechanism controlling the action of GSK3 because signaling pathways phosphorylating its substrates must be active before GSK3 can have any effect on such substrates. The preference of GSK3 for phosphorylating substrates that have been prephosphorylated (or "primed") 4 amino acids C-terminal to the target Ser/Thr is due to the presence of a phosphate binding pocket in GSK3. The phosphate of the primed substrate sits in this pocket and positions the phosphate acceptor site to enable efficient phosphorylation by GSK3.
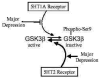
Fig 3. Schematic depiction of the regulation of GSK3 by 5HT1A and 5HT2 receptors and changes that may be associated with major depression
GSK3β is inhibited by phosphorylation of serine-9. This inhibitory phosphorylation is promoted by activation of 5HT1A receptors. However, decreased 5HT1A receptors occur in major depression, so there may be insufficient signaling leading to inhibition of GSK3β in depression. Conversely, activation of 5HT2 receptors cause activation of GSK3β by promoting its dephosphorylation. Increased 5HT2 receptors occur in major depression, suggesting increased activation of GSK3β . Overall, the balance between 5HT1A and 5HT2 receptors contributes to maintaining the normal activity level of GSK3β , and changes in each receptor associated with depression disrupt the 5HT receptor-mediated inhibitory signals that normally control GSK3β , suggesting that the activity of GSK3β is not adequately controlled in major depression.
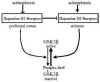
Fig 4. Schematic depiction of the regulation of GSK3 by dopamine D1 and dopamine D2 receptors and changes associated with schizophrenia
GSK3β is inhibited by phosphorylation of serine-9. This inhibitory phosphorylation is promoted by activation of dopamine D1 receptors. However, decreased dopamine D1 receptor activation occurs in schizophrenia, so there may be insufficient signaling leading to inhibition of GSK3β in schizophrenia. Conversely, activation of dopamine D2 receptors cause activation of GSK3β by promoting its dephosphorylation. Increased dopamine D2 receptor activation occurs in schizophrenia, in part by deficient inhibitory inputs from dopamine D1 receptors, suggesting increased activation of GSK3β . Overall, the balance between dopamine D1 and D2 receptors contributes to maintaining the normal activity level of GSK3β , and changes in each receptor associated with schizophrenia disrupt the control of GSK3β .
Similar articles
- Is glycogen synthase kinase-3 a central modulator in mood regulation?
Li X, Jope RS. Li X, et al. Neuropsychopharmacology. 2010 Oct;35(11):2143-54. doi: 10.1038/npp.2010.105. Epub 2010 Jul 28. Neuropsychopharmacology. 2010. PMID: 20668436 Free PMC article. Review. - Glycogen synthase kinase 3 substrates in mood disorders and schizophrenia.
Cole AR. Cole AR. FEBS J. 2013 Nov;280(21):5213-27. doi: 10.1111/febs.12407. Epub 2013 Jul 15. FEBS J. 2013. PMID: 23796137 Review. - Regulation of mouse brain glycogen synthase kinase-3 by atypical antipsychotics.
Li X, Rosborough KM, Friedman AB, Zhu W, Roth KA. Li X, et al. Int J Neuropsychopharmacol. 2007 Feb;10(1):7-19. doi: 10.1017/S1461145706006547. Epub 2006 May 4. Int J Neuropsychopharmacol. 2007. PMID: 16672106 - Blocked inhibitory serine-phosphorylation of glycogen synthase kinase-3alpha/beta impairs in vivo neural precursor cell proliferation.
Eom TY, Jope RS. Eom TY, et al. Biol Psychiatry. 2009 Sep 1;66(5):494-502. doi: 10.1016/j.biopsych.2009.04.015. Epub 2009 Jun 11. Biol Psychiatry. 2009. PMID: 19520363 Free PMC article. - Glycogen synthase kinase-3 (GSK3): inflammation, diseases, and therapeutics.
Jope RS, Yuskaitis CJ, Beurel E. Jope RS, et al. Neurochem Res. 2007 Apr-May;32(4-5):577-95. doi: 10.1007/s11064-006-9128-5. Epub 2006 Aug 30. Neurochem Res. 2007. PMID: 16944320 Free PMC article. Review.
Cited by
- Vortioxetine ameliorates anhedonic-like behaviour and promotes strategic cognitive performance in a rodent touchscreen task.
Martis LS, Højgaard K, Holmes MC, Elfving B, Wiborg O. Martis LS, et al. Sci Rep. 2021 Apr 27;11(1):9113. doi: 10.1038/s41598-021-88462-7. Sci Rep. 2021. PMID: 33907240 Free PMC article. - Glycogen synthase kinase-3 inhibitors as potent therapeutic agents for the treatment of Parkinson disease.
Morales-García JA, Susín C, Alonso-Gil S, Pérez DI, Palomo V, Pérez C, Conde S, Santos A, Gil C, Martínez A, Pérez-Castillo A. Morales-García JA, et al. ACS Chem Neurosci. 2013 Feb 20;4(2):350-60. doi: 10.1021/cn300182g. Epub 2012 Dec 5. ACS Chem Neurosci. 2013. PMID: 23421686 Free PMC article. - Identifying a kinase network regulating FGF14:Nav1.6 complex assembly using split-luciferase complementation.
Hsu WC, Nenov MN, Shavkunov A, Panova N, Zhan M, Laezza F. Hsu WC, et al. PLoS One. 2015 Feb 6;10(2):e0117246. doi: 10.1371/journal.pone.0117246. eCollection 2015. PLoS One. 2015. PMID: 25659151 Free PMC article. - Guanine-Based Purines as an Innovative Target to Treat Major Depressive Disorder.
Almeida RF, Ferreira TP, David CVC, Abreu E Silva PC, Dos Santos SA, Rodrigues ALS, Elisabetsky E. Almeida RF, et al. Front Pharmacol. 2021 Apr 13;12:652130. doi: 10.3389/fphar.2021.652130. eCollection 2021. Front Pharmacol. 2021. PMID: 33927625 Free PMC article. No abstract available. - Chronic over-nutrition and dysregulation of GSK3 in diseases.
Liu X, Yao Z. Liu X, et al. Nutr Metab (Lond). 2016 Aug 4;13:49. doi: 10.1186/s12986-016-0108-8. eCollection 2016. Nutr Metab (Lond). 2016. PMID: 27493677 Free PMC article. Review.
References
- Jope RS. Mol Psychiat. 1999;4:117–128. - PubMed
- Jope RS. Clinical Neuroscience Res. 2004;4:171–179.
- Jope RS, Johnson GVW. Trends Biochem Sci. 2004;29:95–102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AG021045/AG/NIA NIH HHS/United States
- R01 NS037768/NS/NINDS NIH HHS/United States
- R56 MH038752/MH/NIMH NIH HHS/United States
- MH38752/MH/NIMH NIH HHS/United States
- R01 MH038752/MH/NIMH NIH HHS/United States
- R01 AG021045/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical