Interchangeable but essential functions of SNX1 and SNX2 in the association of retromer with endosomes and the trafficking of mannose 6-phosphate receptors - PubMed (original) (raw)
Interchangeable but essential functions of SNX1 and SNX2 in the association of retromer with endosomes and the trafficking of mannose 6-phosphate receptors
Raul Rojas et al. Mol Cell Biol. 2007 Feb.
Abstract
The retromer is a cytosolic/peripheral membrane protein complex that mediates the retrieval of the cation-independent mannose 6-phosphate receptor from endosomes to the trans-Golgi network (TGN) in mammalian cells. Previous studies showed that the mammalian retromer comprises three proteins, named Vps26, Vps29, and Vps35, plus the sorting nexin, SNX1. There is conflicting evidence, however, as to whether a homologous sorting nexin, SNX2, is truly a component of the retromer. In addition, the nature of the subunit interactions and assembly of the mammalian retromer complex are poorly understood. We have addressed these issues by performing biochemical and functional analyses of endogenous retromers in the human cell line HeLa. We found that the mammalian retromer complex consists of two autonomously assembling subcomplexes, namely, a Vps26-Vps29-Vps35 obligate heterotrimer and a SNX1/2 alternative heterodimer or homodimer. The association of Vps26-Vps29-Vps35 with endosomes requires the presence of either SNX1 or SNX2, whereas SNX1/2 can be recruited to endosomes independently of Vps26-Vps29-Vps35. We also found that the presence of either SNX1 or SNX2 is essential for the retrieval of the cation-independent mannose 6-phosphate receptor to the TGN. These observations indicate that the mammalian retromer complex assembles by sequential association of SNX1/2 and Vps26-Vps29-Vps35 subcomplexes on endosomal membranes and that SNX1 and SNX2 play interchangeable but essential roles in retromer structure and function.
Figures
FIG. 1.
Schematic representation of mammalian retromer subunits. The number of amino acids and molecular mass of each protein are indicated. The scheme also indicates the features of the different subunits or their domains.
FIG. 2.
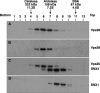
Coimmunoprecipitation of endogenous retromer subunits. (A and B) HeLa cells metabolically labeled with [35S]methionine-cysteine were lysed with 0.5% (vol/vol) Triton X-100 and immunoprecipitated with rabbit anti-Vps26 (A) or rabbit anti-SNX1 (B). Washed immunoprecipitates were eluted by heating at 95°C for 5 min in the presence of 10 mM dithiothreitol and 1% (wt/vol) SDS and later diluted with lysis buffer. Samples were subjected to recapture with rabbit antibodies to Vps26, Vps29, Vps35, SNX1, SNX2, and the β3 subunit of the AP-3 complex as a negative control. Washed immunoprecipitates were analyzed by 12% acrylamide SDS-PAGE followed by fluorography. Two different exposure times are shown in panel A to better document the signal corresponding to Vps29 (this protein migrates as a doublet by SDS-PAGE). (C) HeLa cells were lysed with 0.5% (vol/vol) Triton X-100 and immunoprecipitated with (lane 3) or without (lane 2) a monoclonal antibody to SNX1. After being washed, the immunoprecipitates were eluted in sample buffer and analyzed by 4 to 20% acrylamide gradient SDS-PAGE and immunoblotting with a monoclonal antibody to SNX2. Ten percent of the input lysate was loaded in lane 1 as a control. (D) HeLa cells transfected with plasmids encoding Vps26-Myc (lane 1), CFP-SNX1 (lane 2), and HA-SNX2 (lane 3) were lysed and subjected to SDS-PAGE and immunoblotting with monoclonal antibodies to SNX1, SNX2, and HA and a polyclonal antibody to green fluorescent protein (GFP). The positions of molecular mass markers (in kDa) are indicated on the right in panels A to C. Abbreviations: IP, immunoprecipitation; Ab, antibody; IB, immunoblotting; Endo, endogenous; Ig-HC; immunoglobulin heavy chain; Ig-LC, immunoglobulin light chain.
FIG. 3.
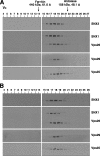
Sedimentation velocity analysis of retromer subunits. Triton X-100 extracts of HeLa cells were fractionated by centrifugation in linear 2 to 10% (wt/vol) sucrose gradients as described in Materials and Methods. Samples representing 4% of the volume of each fraction were analyzed by 4 to 20% acrylamide gradient SDS-PAGE and immunoblotting with antibodies to Vps26 (A), Vps29 (B), and SNX1 (D) or jointly with antibodies to Vps35 and SNX2 (C). The positions in the gradient, molecular masses, and sedimentation coefficients of protein standards are indicated at the top.
FIG. 4.
Gel filtration analysis of retromer subunits. Triton X-100 extracts (A) and cytosol (B) from HeLa cells, prepared as indicated in Materials and Methods, were subjected to gel filtration on a calibrated Superdex 200 HR column. Samples representing 2% of the volume of each fraction were analyzed by 4 to 20% acrylamide gradient SDS-PAGE and immunoblotting with antibodies to each of the retromer subunits. The void volume (_V_0) and the positions, molecular masses, and Stokes radii of standard proteins are indicated at the top.
FIG. 5.
Effects of depleting retromer subunits on the levels and gel filtration behaviors of the other subunits. (A) Triton X-100 extracts from mock-treated cells or cells treated with siRNA to Vps26, Vps29, Vps35, SNX1, SNX2, or SNX1/SNX2 together were subjected to 4 to 20% acrylamide gradient SDS-PAGE and immunoblotting (IB) with antibodies to the indicated proteins. Equal amounts of total protein, as quantified by the bicinchoninic acid assay, were loaded in each lane. (B) Triton X-100 extracts from mock-treated cells or cells treated with siRNA to Vps26 or SNX1 were subjected to gel filtration on a calibrated Superdex 200 HR column. Five percent of the volume of each fraction was analyzed by 4 to 20% acrylamide gradient SDS-PAGE and immunoblotting with a mixture of antibodies to Vps26 and SNX1. The positions, molecular masses, and Stokes radii of standard proteins are indicated at the top. Notice that siRNA depletion of Vps26 did not alter the elution profile of SNX1 (or SNX2 [data not shown]). Similarly, siRNA depletion of SNX1 did not affect the elution properties of Vps26 (or Vps29 and Vps35 [data not shown]).
FIG. 6.
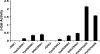
Yeast two-hybrid analysis of interactions between the SNX and Vps subunits of the mammalian retromer. S. cerevisiae strain AH109 was cotransformed with the combinations of SNX and Vps subunits of retromer indicated in the figure. For each pair, the first protein was expressed as a fusion with the Gal4 DNA-binding domain from the pGBKT7 plasmid, while the second protein was expressed as a fusion with the Gal4 transcription activation domain from the pGADT7 plasmid. Minus signs indicate the absence of any retromer subunit in pGBKT7. Interactions were quantified using a liquid β-galactosidase (β-Gal) assay. Activity values (in arbitrary units) are the means ± standard deviations for triplicate determinations. The experiment shown in the figure is representative of four experiments that gave similar results.
FIG. 7.
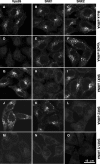
Colocalization of endogenous SNX1, SNX2, and Vps26 in HeLa cells. The intracellular localization of SNX2 (A and D), SNX1 (B and G), and Vps26 (E and H) was assessed in fixed and permeabilized cells by indirect immunofluorescence and confocal microscopy. (A, D, and G) Alexa 488, green channel. (B, E, and H) Alexa 546, red channel. (C, F, and I) Merged images. Yellow indicates colocalization. Examples of foci where proteins colocalize are indicated by arrows.
FIG. 8.
Requirement of SNX1 or SNX2 for association of the Vps26-Vps29-Vps35 subcomplex with endosomal membranes. HeLa cells were treated twice, at 24-h intervals, with an inactive siRNA to Vps35 (mock; A to C) or active siRNA to Vps26 (D to F), SNX1 (G to I), SNX2 (J to L), or SNX1 and SNX2 together (M to O). At 48 h posttreatment, the cellular distributions of Vps26 (A, D, G, J, and M), SNX1 (B, E, H, K, and N), and SNX2 (C, F, I, L, and O) were assessed by indirect immunofluorescence staining using mouse monoclonal antibodies to the corresponding SNX proteins and a rabbit polyclonal antibody to Vps26, followed by FITC-conjugated goat anti-rabbit IgG and Cy5-conjugated goat anti-mouse IgG. Images were captured by confocal microscopy. The same confocal microscope settings were used for imaging of all mock- and siRNA-treated cells.
FIG. 9.
Functional redundancy of SNX1 and SNX2 in sorting of the CI-MPR. (A to F) HeLa cells treated with siRNAs to SNX1 (A and D), SNX2 (B and E), and SNX1 and SNX2 together (C and F) were analyzed by immunofluorescence staining and confocal microscopy. Cells were double stained with rabbit polyclonal antibodies to the corresponding SNX proteins (A to C) and a mouse monoclonal antibody to the CI-MPR (D to F), followed by FITC-conjugated goat anti-rabbit IgG and Cy5-conjugated goat anti-mouse IgG. As internal controls, a minority of cells that did not respond to the siRNA treatment were imaged next to the majority of cells that responded. Arrows point to cells depleted of the indicated SNX protein. (G) Extracts of HeLa cells treated with siRNAs to the proteins indicated at the top were analyzed by 4 to 20% acrylamide gradient SDS-PAGE and immunoblotting (IB) with antibodies to the proteins indicated on the right. Equal amounts of total protein were loaded. Blots were also probed with antibody to α-tubulin as a loading control.
Similar articles
- The retromer component sorting nexin-1 is required for efficient retrograde transport of Shiga toxin from early endosome to the trans Golgi network.
Bujny MV, Popoff V, Johannes L, Cullen PJ. Bujny MV, et al. J Cell Sci. 2007 Jun 15;120(Pt 12):2010-21. doi: 10.1242/jcs.003111. J Cell Sci. 2007. PMID: 17550970 - A loss-of-function screen reveals SNX5 and SNX6 as potential components of the mammalian retromer.
Wassmer T, Attar N, Bujny MV, Oakley J, Traer CJ, Cullen PJ. Wassmer T, et al. J Cell Sci. 2007 Jan 1;120(Pt 1):45-54. doi: 10.1242/jcs.03302. Epub 2006 Dec 5. J Cell Sci. 2007. PMID: 17148574 - An essential role for SNX1 in lysosomal sorting of protease-activated receptor-1: evidence for retromer-, Hrs-, and Tsg101-independent functions of sorting nexins.
Gullapalli A, Wolfe BL, Griffin CT, Magnuson T, Trejo J. Gullapalli A, et al. Mol Biol Cell. 2006 Mar;17(3):1228-38. doi: 10.1091/mbc.e05-09-0899. Epub 2006 Jan 11. Mol Biol Cell. 2006. PMID: 16407403 Free PMC article. - Retromer.
Bonifacino JS, Hurley JH. Bonifacino JS, et al. Curr Opin Cell Biol. 2008 Aug;20(4):427-36. doi: 10.1016/j.ceb.2008.03.009. Epub 2008 May 9. Curr Opin Cell Biol. 2008. PMID: 18472259 Free PMC article. Review. - Updated Insight into the Physiological and Pathological Roles of the Retromer Complex.
Abubakar YS, Zheng W, Olsson S, Zhou J. Abubakar YS, et al. Int J Mol Sci. 2017 Jul 25;18(8):1601. doi: 10.3390/ijms18081601. Int J Mol Sci. 2017. PMID: 28757549 Free PMC article. Review.
Cited by
- Retromer-Mediated Trafficking of Transmembrane Receptors and Transporters.
Klinger SC, Siupka P, Nielsen MS. Klinger SC, et al. Membranes (Basel). 2015 Jul 6;5(3):288-306. doi: 10.3390/membranes5030288. Membranes (Basel). 2015. PMID: 26154780 Free PMC article. Review. - CHC22 clathrin recruitment to the early secretory pathway requires two-site interaction with SNX5 and p115.
Greig J, Bates GT, Yin DI, Briant K, Simonetti B, Cullen PJ, Brodsky FM. Greig J, et al. EMBO J. 2024 Oct;43(19):4298-4323. doi: 10.1038/s44318-024-00198-y. Epub 2024 Aug 19. EMBO J. 2024. PMID: 39160272 Free PMC article. - Retromer oligomerization drives SNX-BAR coat assembly and membrane constriction.
Gopaldass N, De Leo MG, Courtellemont T, Mercier V, Bissig C, Roux A, Mayer A. Gopaldass N, et al. EMBO J. 2023 Jan 16;42(2):e112287. doi: 10.15252/embj.2022112287. EMBO J. 2023. PMID: 36644906 Free PMC article. - Regulation of endosomal clathrin and retromer-mediated endosome to Golgi retrograde transport by the J-domain protein RME-8.
Shi A, Sun L, Banerjee R, Tobin M, Zhang Y, Grant BD. Shi A, et al. EMBO J. 2009 Nov 4;28(21):3290-302. doi: 10.1038/emboj.2009.272. Epub 2009 Sep 17. EMBO J. 2009. PMID: 19763082 Free PMC article. - A syntaxin 10-SNARE complex distinguishes two distinct transport routes from endosomes to the trans-Golgi in human cells.
Ganley IG, Espinosa E, Pfeffer SR. Ganley IG, et al. J Cell Biol. 2008 Jan 14;180(1):159-72. doi: 10.1083/jcb.200707136. J Cell Biol. 2008. PMID: 18195106 Free PMC article.
References
- Barr, V. A., S. A. Phillips, S. I. Taylor, and C. R. Haft. 2000. Overexpression of a novel sorting nexin, SNX15, affects endosome morphology and protein trafficking. Traffic 1:904-916. - PubMed
- Bonifacino, J. S., and E. C. Dell'Angelica. 1998. Immunoprecipitation, p. 7.2.1-7.2.21. In J. S. Bonifacino, M. Dasso, J. B. Harford, J. Lippincott-Schwartz, and K. Yamada (ed.), Current protocols in cell biology. John Wiley & Sons, New York, NY.
- Bonifacino, J. S., and B. S. Glick. 2004. The mechanisms of vesicle budding and fusion. Cell 116:153-166. - PubMed
- Bonifacino, J. S., and R. Rojas. 2006. Retrograde transport from endosomes to the trans-Golgi network. Rev. Mol. Cell Biol. 7:568-579. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous