Role for the Ssu72 C-terminal domain phosphatase in RNA polymerase II transcription elongation - PubMed (original) (raw)
Role for the Ssu72 C-terminal domain phosphatase in RNA polymerase II transcription elongation
Mariela Reyes-Reyes et al. Mol Cell Biol. 2007 Feb.
Abstract
The RNA polymerase II (RNAP II) transcription cycle is accompanied by changes in the phosphorylation status of the C-terminal domain (CTD), a reiterated heptapeptide sequence (Y(1)S(2)P(3)T(4)S(5)P(6)S(7)) present at the C terminus of the largest RNAP II subunit. One of the enzymes involved in this process is Ssu72, a CTD phosphatase with specificity for serine-5-P. Here we report that the ssu72-2-encoded Ssu72-R129A protein is catalytically impaired in vitro and that the ssu72-2 mutant accumulates the serine-5-P form of RNAP II in vivo. An in vitro transcription system derived from the ssu72-2 mutant exhibits impaired elongation efficiency. Mutations in RPB1 and RPB2, the genes encoding the two largest subunits of RNAP II, were identified as suppressors of ssu72-2. The rpb1-1001 suppressor encodes an R1281A replacement, whereas rpb2-1001 encodes an R983G replacement. This information led us to identify the previously defined rpb2-4 and rpb2-10 alleles, which encode catalytically slow forms of RNAP II, as additional suppressors of ssu72-2. Furthermore, deletion of SPT4, which encodes a subunit of the Spt4-Spt5 early elongation complex, also suppresses ssu72-2, whereas the spt5-242 allele is suppressed by rpb2-1001. These results define Ssu72 as a transcription elongation factor. We propose a model in which Ssu72 catalyzes serine-5-P dephosphorylation subsequent to addition of the 7-methylguanosine cap on pre-mRNA in a manner that facilitates the RNAP II transition into the elongation stage of the transcription cycle.
Figures
FIG. 1.
The Ssu72-R129A protein exhibits impaired phosphatase activity in vitro and in vivo. (A) Purified, recombinant GST-Ssu72 and GST-Ssu72-R129A proteins were assayed for phosphatase activity by production of _p_-nitrophenol (spectroscopic absorbance at 410 nm) from pNPP as described by Ganem et al. (14) using 5 μg of protein. (B) RNAP II CTD serine-5-P accumulates in the _ssu72_-2 mutant at the restrictive temperature. Cell extracts were prepared from isogenic wild-type (LRB535), _ssu72_-2 (YZS84), and _ssu72 rpb1_-1001 (YMH931) strains that had been incubated at the permissive (30°C) or restrictive (37°C) temperature for 60 min, followed by Western blot analysis using antibodies directed against the unphosphorylated form of RNAP II (8WG16), RNAP IIO serine-5-P (H14), or Ssu72. Antiserum against the Rpa1 protein served as a loading control.
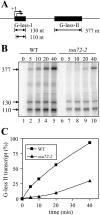
FIG. 2.
Loss of Ssu72 function adversely affects RNAP II transcription in vitro. (A) Schematic depiction of the double G-less cassette (pSLCYC-L) used as template DNA. Transcription initiates at either of two sites within the G-less I cassette, +1 or +20, denoted by the arrows. Following in vitro transcription, RNase T1 digestion of transcripts extending beyond the G-less I cassette yield 110-nt and 130-nt products, whereas the 377-nt product is derived from transcripts that initiate at either +1 or +20 and extend beyond the G-less II cassette (32, 33). (B) In vitro transcription reactions were carried out for the indicated times using whole-cell extracts derived from the SSU72 wild-type (LRB535) or _ssu72_-2 (YZS84) mutant strains, digested with RNase T1, and resolved by electrophoresis in a 6% polyacrylamide-urea gel. (C) Efficiency of elongation determined from the in vitro transcription data in panel B. Radioactivity ([α-32P]UTP) incorporated into the 110-nt, 130-nt, and 377-nt transcripts was quantified using a PhosphorImager and normalized to the uridine content of each G-less transcript. The percentage of G-less II transcripts (377 nt) relative to total transcripts, defined by G-less I transcripts (110 nt plus 130 nt), is plotted at each time point for the wild-type and mutant strains.
FIG. 3.
Growth defects associated with the _ssu72_-2 mutation and its suppressors. Tenfold serial dilutions of the wild-type (SSU72), primary mutant (_ssu72_-2), and the five independent suppressor (supA to supE) strains were spotted onto the indicated medium. Plates were photographed following incubation for 2 (YPD, 30°C) or 3 (YPD, 37°C; +Ino; −Ino) days. Strains are LRB535 (row 1), YZS84 (row 2), YMH930 (row 3), YMH931 (row 4), YMH932 (row 5), YMH933 (row 6), and YMH934 (row 7). The complete genotype of each strain is indicated in Table 1. Growth media are defined in Materials and Methods.
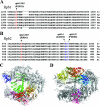
FIG. 4.
The _ssu72_-2 suppressors encode single amino acid replacements in RNAP II. (A) The _rpb1_-1001 allele encodes a leucine replacement of the phylogenetically conserved arginine at position 1281 (R1281L) within the “cleft” domain of Rpb1. The sequence alignment depicts the “cleft,” which lies just C terminal to the “jaw” domain of Rpb1 (11). Sequences are from S. cerevisiae (Sc), S. pombe (Sp), D. melanogaster (Dm), Homo sapiens (Hs), Mus musculus (Mm), C. elegans (Ce), and A. thaliana (At). (B) The _rpb2_-1001 allele encodes a glycine replacement of the phylogenetically invariant arginine at position 983 (R983G) within the “hybrid binding” domain of Rpb2. The sequence alignment depicts the region of the hybrid binding domain just C terminal to the “wall” of RNAP II (11). Also shown are the previously defined A1016T and P1018S replacements encoded by the _rpb2_-4 and _rpb2_-10 alleles, respectively (51, 59). (C, D) Three-dimensional structures of RNAP II, highlighting domains of Rpb1 (C) and Rpb2 (D). The positions of the _rpb1_-_1001_-encoded R1281L and _rpb2_-_1001_-encoded R983G replacements are depicted.
FIG. 5.
Slowing the rate of transcription elongation suppresses the _ssu72_-2 Tsm− phenotype. (A) Effects of rpb2 mutations. Tenfold serial dilutions of the wild-type (LRB535, row 1), _ssu72_-2 (YZS84, row 2), and _ssu72_-_2 rpb2_-1001 (YMH931, row 7) strains, as well as the _ssu72_-2 plasmid shuffle strains harboring plasmids carrying the indicated rpb2 alleles, were spotted onto YPD medium and incubated at the indicated temperatures for either 2 (30°C) or 3 (37°C) days and photographed. The strains in rows 3 to 6 are YMH936, YMH937, YMH943, and YMH938, respectively. The complete genotype of each strain is indicated in Table 1. (B) Effects of 6-AU. Tenfold serial dilutions of the wild-type (LRB535) and _ssu72_-2 (YZS84) strains were spotted onto YPD medium containing the indicated concentrations of 6-AU and incubated at either 30°C (2 days) or 37°C (3 days) and photographed.
FIG. 6.
Genetic interactions among Ssu72, Rpb2, and the Spt4-Spt5 complex. (A) The _spt4_Δ deletion suppresses the _ssu72_-2 Tsm− phenotype. Tenfold serial dilutions of wild-type (LRB535), _dst1_Δ (YMH938), _spt4_Δ (YMH940), _ssu72_-2 (YZS84), _ssu72_-_2 dst1_Δ (YMH939), _ssu72_-_2 spt4_Δ (YMH941) were spotted onto YPD medium and incubated for 2 (30°C) or 3 (37°C) days and photographed. The DST1 gene encodes the transcription elongation factor TFIIS; in contrast to _spt4_Δ, the _dst1_Δ deletion failed to suppress the _ssu72_-2 Tsm− phenotype. (B) The _rpb2_-1001 allele suppresses the _spt5_-242 Csm− phenotype. Tenfold serial dilutions of the _spt5_-242 (GHY339) and _spt5_-_242 rpb2_-1001 (YMH942) strains were spotted on YPD medium and incubated for 2 (30°C) or 5 (16°C) days and photographed.
FIG. 7.
Model depicting Ssu72-mediated serine-5-P dephosphorylation at different stages of the transcription cycle. Transcription initiation coincides with phosphorylation of serine-5 of the RNAP II CTD (step 1). The Spt4-Spt5 complex acts early in the transcription cycle (step 2) (35) and facilitates recruitment of the capping machinery (CE) (step 3) (8, 31, 40, 55, 60, 72). We propose that Ssu72 catalyzes partial serine-5-P dephosphorylation subsequent to capping (depicted by red ball at 5′-end of nascent transcript [red line]) and facilitates the transition from initiation to elongation (step 4), perhaps by promoting displacement of the capping machinery from RNAP II (43). Additional Ssu72-catalyzed serine-5-P dephosphorylation might occur during early elongation (step 5) as a prerequisite to Ctk1-mediated serine-2 phosphorylation (step 6) (31). Ssu72 also affects the elongation-termination transition (12, 14, 24, 66) and, along with the Fcp1 serine-2-P phosphatase, restores the initiation-competent, hypophosphorylated form of RNAP II (step 7). This hypophosphorylated form of RNAP II (IIA) is now competent for assembly into an initiation complex for subsequent rounds of transcription (step 8). See Discussion for details.
Similar articles
- Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.
Salisbury L, Baraitser L. Salisbury L, et al. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review. - Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.
Lee CC, Chen CW, Yen HK, Lin YP, Lai CY, Wang JL, Groot OQ, Janssen SJ, Schwab JH, Hsu FM, Lin WH. Lee CC, et al. Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924 - Yeast Rpb9 plays an important role in ubiquitylation and degradation of Rpb1 in response to UV-induced DNA damage.
Chen X, Ruggiero C, Li S. Chen X, et al. Mol Cell Biol. 2007 Jul;27(13):4617-25. doi: 10.1128/MCB.00404-07. Epub 2007 Apr 23. Mol Cell Biol. 2007. PMID: 17452455 Free PMC article. - Genetic and structural analysis of the essential fission yeast RNA polymerase II CTD phosphatase Fcp1.
Schwer B, Ghosh A, Sanchez AM, Lima CD, Shuman S. Schwer B, et al. RNA. 2015 Jun;21(6):1135-46. doi: 10.1261/rna.050286.115. Epub 2015 Apr 16. RNA. 2015. PMID: 25883047 Free PMC article. - The CDK9-SPT5 Axis in Control of Transcription Elongation by RNAPII.
Sun R, Fisher RP. Sun R, et al. J Mol Biol. 2025 Jan 1;437(1):168746. doi: 10.1016/j.jmb.2024.168746. Epub 2024 Aug 13. J Mol Biol. 2025. PMID: 39147127 Review.
Cited by
- Control of eukaryotic gene expression: gene loops and transcriptional memory.
Hampsey M, Singh BN, Ansari A, Lainé JP, Krishnamurthy S. Hampsey M, et al. Adv Enzyme Regul. 2011;51(1):118-25. doi: 10.1016/j.advenzreg.2010.10.001. Epub 2010 Oct 29. Adv Enzyme Regul. 2011. PMID: 21036187 Free PMC article. Review. - New insights into the role of TFIIB in transcription initiation.
Wang Y, Roberts SG. Wang Y, et al. Transcription. 2010 Nov;1(3):126-129. doi: 10.4161/trns.1.3.12900. Epub 2010 Jul 6. Transcription. 2010. PMID: 21326885 Free PMC article. - Rpb4/7 facilitates RNA polymerase II CTD dephosphorylation.
Allepuz-Fuster P, Martínez-Fernández V, Garrido-Godino AI, Alonso-Aguado S, Hanes SD, Navarro F, Calvo O. Allepuz-Fuster P, et al. Nucleic Acids Res. 2014 Dec 16;42(22):13674-88. doi: 10.1093/nar/gku1227. Nucleic Acids Res. 2014. PMID: 25416796 Free PMC article. - Ser7 phosphorylation of the CTD recruits the RPAP2 Ser5 phosphatase to snRNA genes.
Egloff S, Zaborowska J, Laitem C, Kiss T, Murphy S. Egloff S, et al. Mol Cell. 2012 Jan 13;45(1):111-22. doi: 10.1016/j.molcel.2011.11.006. Epub 2011 Dec 1. Mol Cell. 2012. PMID: 22137580 Free PMC article. - RNA polymerase II plays an active role in the formation of gene loops through the Rpb4 subunit.
Allepuz-Fuster P, O'Brien MJ, González-Polo N, Pereira B, Dhoondia Z, Ansari A, Calvo O. Allepuz-Fuster P, et al. Nucleic Acids Res. 2019 Sep 26;47(17):8975-8987. doi: 10.1093/nar/gkz597. Nucleic Acids Res. 2019. PMID: 31304538 Free PMC article.
References
- Bentley, D. 2002. The mRNA assembly line: transcription and processing machines in the same factory. Curr. Opin. Cell Biol. 14:336-342. - PubMed
- Berroteran, R. W., D. E. Ware, and M. Hampsey. 1994. The sua8 suppressors of Saccharomyces cerevisiae encode replacements of conserved residues within the largest subunit of RNA polymerase II and affect transcription start site selection similarly to sua7 (TFIIB) mutations. Mol. Cell. Biol. 14:226-237. - PMC - PubMed
- Boeke, J. D., F. Lacroute, and G. R. Fink. 1984. A positive selection for mutants lacking orotidine-5′-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol. Gen. Genet. 197:345-346. - PubMed
- Buratowski, S. 2005. Connections between mRNA 3′ end processing and transcription termination. Curr. Opin. Cell Biol. 17:257-261. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R25 GM058389/GM/NIGMS NIH HHS/United States
- R01 GM039484/GM/NIGMS NIH HHS/United States
- GM 55145/GM/NIGMS NIH HHS/United States
- T32 GM008360/GM/NIGMS NIH HHS/United States
- R01 GM 68887/GM/NIGMS NIH HHS/United States
- R01 GM068887/GM/NIGMS NIH HHS/United States
- R25 GM055145/GM/NIGMS NIH HHS/United States
- R01 GM 39484/GM/NIGMS NIH HHS/United States
- GM 008360/GM/NIGMS NIH HHS/United States
- GM 58389/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous