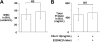Thrombin-activatable procarboxypeptidase B regulates activated complement C5a in vivo - PubMed (original) (raw)
Thrombin-activatable procarboxypeptidase B regulates activated complement C5a in vivo
Toshihiko Nishimura et al. Blood. 2007.
Erratum in
- Blood. 2007 May 1;109(9):3632. Piliposky, Adrian M [corrected to Piliponsky, Adrian M]
Abstract
Plasma procarboxypeptidase B (proCPB) is activated by the endothelial thrombin-thrombomodulin [corrected] complex. Activated proCPB [corrected] (CPB) functions as a fibrinolysis inhibitor, but it may play a broader role by inactivating inflammatory mediators. To test this hypothesis, C5a-induced alveolitis was studied in wild-type (WT) and proCPB-deficient mice (proCPB-/-). C5a-induced alveolitis, as measured by cell counts and total protein contents in bronchoalveolar lavage fluids, was markedly enhanced in the proCPB-/- mice. E229K thrombin, a thrombin mutant with minimal clotting activity but retaining its ability to activate protein C and proCPB, attenuated C5a-induced alveolitis in WT but not in proCPB-/- mice, indicating that its beneficial effect is mediated primarily by its activation of proCPB. Lung tissue histology confirmed these cellular inflammatory responses. Delayed administration of E229K thrombin after the C5a instillation was ineffective in reducing alveolitis in WT mice, suggesting that the beneficial effect of E229K thrombin is due to the direct inhibition of C5a by CPB. Our studies show that thrombin-activatable proCPB, in addition to its role in fibrinolysis, has intrinsic anti-inflammatory functions. Its activation, along with protein C, by the endothelial thrombin-TM complex represents a homeostatic response to counteract the inflammatory mediators generated at the site of vascular injury.
Figures
Figure 1
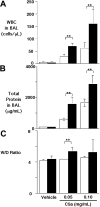
C5a-induced alveolitis in WT and proCPB−/− mice. C5a at 2 concentrations or saline control (vehicle) were instilled endotracheally in WT (□) or proCPB−/− (■) mice. Six hours later, BAL was performed, and total WBC counts (A) and total protein contents (B) in the BAL fluids were determined. (C) The wet-to-dry weight ratio of the right upper lobe was determined as described in “Materials and Methods” (n = 6 in each group; ** P < .05). Data are presented as mean ± SD.
Figure 2
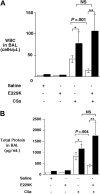
E229K thrombin attenuated C5a-induced alveolitis in WT but not in proCPB−/− mice. C5a (0.1 mg/mL) or saline control was instilled endotracheally in WT (□) or proCPB−/− (■) mice. E229K thrombin or saline control was administered intravenously into the right jugular vein 5 minutes before the C5a instillation. Six hours later, BAL was performed and total WBC counts (A) and protein contents (B) in the BAL fluids were determined. (n = 6 in each group). Data are presented as mean ± SD. *P < .05; **P < .01.
Figure 3
C5a-induced alveolitis in WT and proCPB−/− mice. Representative lung tissue samples of C5a-induced alveolitis from WT mice in the absence (A) or presence (B) of pretreatment with E229K thrombin and from proCPB−/− mice in the absence (C) or presence (D) of pretreatment with E229K thrombin. Black arrows show macrophages. Images at 200× total magnification were visualized using a Zeiss Axiovert 40 CFL microscope equipped with an A-plan 20×/0.30 numerical aperture ph1 objective lens (Zeiss, Dublin, CA). Images were captured and rendered using a Zeiss AxioCam camera and AxioVision AC software.
Figure 4
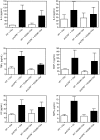
Chemokine and cytokine levels in BAL fluids. Cytokine levels for IL-5, IL-6, TNFα, and MCP-1 were determined from BAL fluids clarified by centrifugation by flow cytometry using the BD mouse Th1/Th2 and BD mouse inflammation cytometric bead array (CBA) kits using the methods outlined by the manufacturers. The levels of mouse KC and MIP-3α were determined by sandwich ELISA according to the manufacturer's instructions. Data are presented as mean ± SD.
Figure 5
Delayed administration of E229K thrombin did not rescue C5a-induced alveolitis in WT mice. E229K thrombin or saline control was administered intravenously 2 hours after the initiation of C5a (0.10 mg/mL) induced alveolitis in this study (n = 6 in each group). Data are presented as mean ± SD.
Similar articles
- Regulation of tissue inflammation by thrombin-activatable carboxypeptidase B (or TAFI).
Leung LL, Myles T, Nishimura T, Song JJ, Robinson WH. Leung LL, et al. Mol Immunol. 2008 Oct;45(16):4080-3. doi: 10.1016/j.molimm.2008.07.010. Epub 2008 Aug 15. Mol Immunol. 2008. PMID: 18706698 Free PMC article. Review. - Regulation of tissue inflammation by thrombin-activatable carboxypeptidase B (or TAFI).
Leung LL, Nishimura T, Myles T. Leung LL, et al. Adv Exp Med Biol. 2008;632:61-9. Adv Exp Med Biol. 2008. PMID: 19025114 Review. - Thrombin activatable fibrinolysis inhibitor, a potential regulator of vascular inflammation.
Myles T, Nishimura T, Yun TH, Nagashima M, Morser J, Patterson AJ, Pearl RG, Leung LL. Myles T, et al. J Biol Chem. 2003 Dec 19;278(51):51059-67. doi: 10.1074/jbc.M306977200. Epub 2003 Oct 2. J Biol Chem. 2003. PMID: 14525995 - Thrombin-activatable fibrinolysis inhibitor protects against acute lung injury by inhibiting the complement system.
Naito M, Taguchi O, Kobayashi T, Takagi T, D'Alessandro-Gabazza CN, Matsushima Y, Boveda-Ruiz D, Gil-Bernabe P, Matsumoto T, Chelakkot-Govindalayathil AL, Toda M, Yasukawa A, Hataji O, Morser J, Takei Y, Gabazza EC. Naito M, et al. Am J Respir Cell Mol Biol. 2013 Oct;49(4):646-53. doi: 10.1165/rcmb.2012-0454OC. Am J Respir Cell Mol Biol. 2013. PMID: 23721130 - Enhanced abdominal aortic aneurysm formation in thrombin-activatable procarboxypeptidase B-deficient mice.
Schultz G, Tedesco MM, Sho E, Nishimura T, Sharif S, Du X, Myles T, Morser J, Dalman RL, Leung LL. Schultz G, et al. Arterioscler Thromb Vasc Biol. 2010 Jul;30(7):1363-70. doi: 10.1161/ATVBAHA.109.202259. Epub 2010 Apr 29. Arterioscler Thromb Vasc Biol. 2010. PMID: 20431069
Cited by
- Regulation of tissue inflammation by thrombin-activatable carboxypeptidase B (or TAFI).
Leung LL, Myles T, Nishimura T, Song JJ, Robinson WH. Leung LL, et al. Mol Immunol. 2008 Oct;45(16):4080-3. doi: 10.1016/j.molimm.2008.07.010. Epub 2008 Aug 15. Mol Immunol. 2008. PMID: 18706698 Free PMC article. Review. - Thrombomodulin mutations in atypical hemolytic-uremic syndrome.
Delvaeye M, Noris M, De Vriese A, Esmon CT, Esmon NL, Ferrell G, Del-Favero J, Plaisance S, Claes B, Lambrechts D, Zoja C, Remuzzi G, Conway EM. Delvaeye M, et al. N Engl J Med. 2009 Jul 23;361(4):345-57. doi: 10.1056/NEJMoa0810739. N Engl J Med. 2009. PMID: 19625716 Free PMC article. - Carboxypeptidase B2 and N play different roles in regulation of activated complements C3a and C5a in mice.
Morser J, Shao Z, Nishimura T, Zhou Q, Zhao L, Higgins J, Leung LLK. Morser J, et al. J Thromb Haemost. 2018 May;16(5):991-1002. doi: 10.1111/jth.13964. Epub 2018 Apr 6. J Thromb Haemost. 2018. PMID: 29383821 Free PMC article. - Regulation of chemerin bioactivity by plasma carboxypeptidase N, carboxypeptidase B (activated thrombin-activable fibrinolysis inhibitor), and platelets.
Du XY, Zabel BA, Myles T, Allen SJ, Handel TM, Lee PP, Butcher EC, Leung LL. Du XY, et al. J Biol Chem. 2009 Jan 9;284(2):751-8. doi: 10.1074/jbc.M805000200. Epub 2008 Nov 14. J Biol Chem. 2009. PMID: 19010784 Free PMC article. - Plasma carboxypeptidase B downregulates inflammatory responses in autoimmune arthritis.
Song JJ, Hwang I, Cho KH, Garcia MA, Kim AJ, Wang TH, Lindstrom TM, Lee AT, Nishimura T, Zhao L, Morser J, Nesheim M, Goodman SB, Lee DM, Bridges SL Jr; Consortium for the Longitudinal Evaluation of African Americans with Early Rheumatoid Arthritis (CLEAR) Registry; Gregersen PK, Leung LL, Robinson WH. Song JJ, et al. J Clin Invest. 2011 Sep;121(9):3517-27. doi: 10.1172/JCI46387. Epub 2011 Aug 1. J Clin Invest. 2011. PMID: 21804193 Free PMC article.
References
- Walport MJ. Complement. N Engl J Med. 2001;344:1058–1066. - PubMed
- Guo RF, Ward PA. Role of C5a in inflammatory responses. Annu Rev Immunol. 2005;23:821–852. - PubMed
- Haviland DL, McCoy RL, Whitehead WT, et al. Cellular expression of the C5a anaphylatoxin receptor (C5aR): demonstration of C5aR on nonmyeloid cells of the liver and lung. J Immunol. 1995;154:1861–1869. - PubMed
- Schieferdecker HL, Schlaf G, Jungermann K, Gotze O. Functions of anaphylatoxin C5a in rat liver: direct and indirect actions on nonparenchymal and parenchymal cells. Int Immunopharmacol. 2001;1:469–481. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources