The c-Jun N-terminal kinase activator dual leucine zipper kinase regulates axon growth and neuronal migration in the developing cerebral cortex - PubMed (original) (raw)
The c-Jun N-terminal kinase activator dual leucine zipper kinase regulates axon growth and neuronal migration in the developing cerebral cortex
Syu-ichi Hirai et al. J Neurosci. 2006.
Abstract
Mammalian corticogenesis substantially depends on migration and axon projection of newborn neurons that are coordinated by a yet unidentified molecular mechanism. Dual leucine zipper kinase (DLK) induces activation of c-Jun N-terminal kinase (JNK), a molecule that regulates morphogenesis in various organisms. We show here, using gene targeting in mice, that DLK is indispensable for establishing axon tracts, especially those originating from neocortical pyramidal neurons of the cerebrum. Direct and quantitative analysis of radial migration of pyramidal neurons using slice culture and a time-lapse imaging system revealed that acceleration around the subplate was affected by DLK gene disruption and by administration of a JNK inhibitor. Phosphorylation of JNK substrates, including c-Jun and doublecortin, and of JNK itself at the activation loop were partially affected in brains of DLK-deficient mouse embryos. These data suggest that DLK plays a significant role in the coordinated regulation of radial migration and axon projection by modulating JNK activity.
Figures
Figure 1.
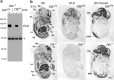
Generation of DLK mutant mice. a, Schematic representation of the DLK gene targeting strategy. The exon encoding 224 N-terminal amino acids including the ATP binding motif of the kinase domain (amino acid sequence is indicated at the top) was first replaced with tandemly arrayed IRES–LacZ and a floxed MC1–neo cassette (Targeted allele). The MC1–neo cassette was then removed (Targeted allele Δneo) by crossing with CAG–Cre gene transgenic mice (see Materials and Methods). DLK-CT indicates the region of DLK protein used as antigenic sequence for polyclonal and monoclonal antibodies. The homologous region included in the targeting vector is also shown with a gray line. FC2, BB1R, and L3 indicate annealing sites of primers used for genotype analysis. B, _Bam_HI; E, _Eco_RI; Ev, _Eco_RV. b, Southern blot analysis of genomic DNA obtained from liver of wild-type or mutant embryos. Regions of genomic DNA corresponding to the cDNA probes used for Southern blot analysis (BS and EE) are shown in a, at the bottom. Arrows indicate sizes of DNA fragments hybridized with each probe. EE probe detected 9.1 kb _Bam_HI fragment of targeted allele including the neo cassette and 7.7 kb _Bam_HI fragment of the targeted allele without the neo cassette.
Figure 2.
Loss of DLK expression in homozygous mutant mice. a, Western blot analysis of DLK protein immunoprecipitated from E16 brain lysates of wild-type (Dlk+/+), heterozygous mutant (Dlk+/−), and homozygous mutant (Dlk_−/−) mice. A mouse monoclonal antibody raised against the C-terminal part of the DLK protein (Fig. 1_a) was used for immunoprecipitation, and a rabbit polyclonal antibody raised against the C-terminal part of DLK protein was used for Western blot analysis. No residual protein reacting with these antibodies was detected in lysates prepared from brains of homozygous mutants. b, Sagittal sections of wild-type (Dlk+/+) or homozygous mutant (_Dlk_−/−) E14 embryos were stained with hematoxylin and eosin (HE), DLK polyclonal antibody, or βIII-tubulin, as indicated. Positive DLK antibody staining of neural tissues observed in sections of wild-type embryos was not visible in mutants, whereas generations of neural tissues including CNS and peripheral nervous system and non-neural tissues were not affected. Tc, Telencephalon; Mc, mesencephalon; Cb, cerebellar anlage; Sp, spinal cord; Ht, heart; Lu, lung; Lv, liver; In, intestine; St, stomach; Kd, kidney; CG, cervical ganglion; DRG, dorsal root ganglion. Scale bar, 1 mm.
Figure 3.
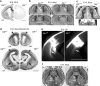
Loss of DLK affects fiber tract development. a, DLK protein in coronal sections of the cerebrum prepared from heterozygous (left hemisphere) and homozygous mutant (right hemisphere) embryos were stained with the anti-DLK polyclonal antibody. b, Coronal sections of wild-type (Dlk+/+) or homozygous mutant (_Dlk_−/−) head portions at E19 were stained with cresyl violet. Two sections (top and bottom) separated by ∼700 μm from each other revealed commissure neuron dysgenesis. The corpus callosum was particularly reduced in size at the front part (top row) in mutants. As for the anterior commissure, the anterior part was hardly seen, and the posterior part was reduced in mutants. c, The structure of olfactory bulb shown by Nissl staining (top row) and DiI labeling of lateral olfactory tract (bottom rows). Although the layer structure of olfactory bulb was preserved in homozygous mutants (_Dlk_−/−), thickness of glomerular layer (arrows) and lateral olfactory tract (arrowheads) was reduced. d, Rostrocaudal sections of heterozygous mutant (Dlk+/−) or homozygous mutant (_Dlk_−/−) brains at E18 were stained with a neurofilament antibody. In homozygous mutant brains, a reduction of neural fibers in the cingulum (arrowheads) and internal capsule (arrows) was obvious. Neural fibers along the hippocampus were apparently not affected. e, DiI labeling of ventrally extended axons from cortical neurons (arrows), which are reduced in mutant (_Dlk_−/−). f, Structures of the hippocampus and its associated neural fibers are shown as horizontal sections stained with cresyl violet. Structures of the psalterium, fimbria, and the hippocampus itself were indistinguishable between wild-types and mutants. CC, Corpus callosum; aAC, anterior part of the anterior commissure; pAC, posterior part of the anterior commissure; OB, olfactory bulb; HI, hippocampus; LOT, lateral olfactory tract; FIM, fimbria; PS, psalterium. Asterisks indicate DiI injection sites. Scale bars, 1 mm.
Figure 4.
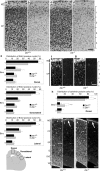
Radial migration in the neocortical region is affected by disruption of the DLK gene. a-d, BrdU was administered at E15, and the brain was fixed at E19. Coronal sections of wild type (a, b) or mutant (c, d) dorsal neocortex beneath bregma, stained with cresyl violet (a, c) or an anti-BrdU antibody (b, d). e, To quantify the distribution of BrdU-positive nuclei, the cortical plate was divided into four equal bins from the pial side to the ventricular side. The number of nuclei in each bin was counted and are shown as percentages of the total nuclei number in the cortical plate. Coronal sections of dorsal, dorsolateral, and lateral neocortices beneath bregma were used for analysis. *p < 0.05; n = 3. f, g, Immunofluorescent staining (cyanine 3) of coronal sections of E19 dorsal neocortex with anti-calretinin. Panels accompanied by f and g show nonspecific interaction of secondary antibody (Alexa488) by blood cells. Calretinin-positive neurons in the cortical plate were more dispersed in mutants (g). h, Numbers of calretinin-positive cells in each bin were counted and are shown as percentages of the total count. The deep layer of cortical plate enriched with calretinin-positive neural fibers was excluded from this analysis. **p < 0.01, ***p < 0.001; n = 8 sections. i–l, Immunofluorescent staining of coronal sections of the dorsal neocortex with anti-vimentin (i, k) and anti-reelin (j, l) antibodies. Radial glial architecture and reelin expression (arrow) in wild types (i, j) were preserved in mutants (k, l). The bright scattered signals correspond to nonspecific staining of blood cells by the secondary antibodies. Arrows indicate white belts located at the marginal zone. MZ, Marginal zone; VZ, ventricular zone. Scale bars, 50 μm.
Figure 5.
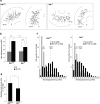
Acceleration of radial migration at the subplate/cortical plate region is affected by disruption of the DLK gene. a, For quantitative analysis of radial migration, migration of individual GFP-labeled cells in slice cultures of E16.5 cortices were traced on a movie screen (for details, see Results). The starting points for each cell are shown by dots, and net displacement (distance and direction) during 6 h is shown by linear lines. b, The average values of net displacement of GFP-labeled cells located in different areas including the IZ and the CP were first calculated for each cultured slice. Then, averages and SDs of net displacement for wild-type slices (n = 11) and mutant slices (n = 14) were calculated and are shown graphically. Error bars indicate SDs. ***p < 0.001. c, The frequency distributions of net displacement for individual cells are shown as histograms. Median values for cells in different areas are shown with dotted lines. d, The percentage of migrating cells in SP–CP region. Cells whose soma changed position within 30 min were counted as migrating cells. Values were calculated for each slice, and average and SD for wild-type or mutant slices are shown graphically. *p < 0.01.
Figure 6.
Loss of DLK expression results in a decrease in JNK activity. a, DLK protein level in whole brain (E16) extracts was quantified by Western blotting using a rabbit polyclonal antibody raised against the C-terminal part of DLK. DLK protein level was significantly reduced in heterozygous mutants (***p < 0.001) and was absent in homozygous mutants. The faint bands detected in the lysate from homozygous mutants corresponded to nonspecific interaction of the secondary antibody, which were removed by immunoprecipitation using the anti-DLK monoclonal antibody (Fig. 2_a_). b, JNK activity was monitored by Western blotting using an anti-active (pThr–Pro–pTyr) JNK antibody. Both the p46 fraction and the p55 fraction of active JNK were significantly decreased in homozygous mutants. The total amount of p46 JNK was also quantified and was found to be slightly increased in mutants. **p < 0.01, ***p < 0.001; n = 5.
Figure 7.
Phosphorylation levels of JNK substrates decreased in E16 brains of DLK homozygous mutants. a, Protein levels of c-Jun phosphorylated at Ser73 and c-Jun in total were quantified by Western blot analysis. The amount of phospho-c-Jun was decreased in homozygous mutants to less than half that of wild-type embryos. b, Protein levels of DCX phosphorylated at Thr331 and Ser334 and DCX in total were quantified by Western blotting. The amount of the phospho-DCX was significantly decreased in homozygous mutants, although the difference with wild types was not significant. c, The phosphorylation level of MAP2c was estimated from the ratio of mobility-delayed bands (pMAP2c) in total MAP2c bands. These mobility delayed bands were detected with an anti-phospho-Thr–Pro antibody and disappeared after phosphatase treatment in vitro (supplemental Fig. S2, available at
as supplemental material). The relative amount of pMAP2c was also slightly decreased in homozygous mutants. d, No significant change was observed in tau protein level or its phosphorylation level when calculated by Western blotting using an anti-tau antibody, an anti-dephosphorylated tau antibody (tau-1), and an anti-tau protein phosphorylated at Ser202 (AT-8). *p < 0.05, **p < 0.01, ***p < 0.001, respectively; n = 5.
Figure 8.
Inhibition of JNK activity significantly impairs radial migration in cortical slice cultures. a, The average values of net displacement of GFP-labeled cells located in different areas, i.e., in the ventricular side and the pial side (for area definition, see supplemental Fig. S6, available at
as supplemental material), were first calculated for each cultured slice. Then, averages and SDs of net displacement for slices treated with vehicle (DMSO; n = 12) or JNK inhibitor (SP600125; n = 12) were calculated and are shown graphically. Error bars indicate SDs. ***p < 0.001. b, The frequency distributions of net displacement of individual cells are shown using histograms. Median values for cells in different areas are shown with dotted lines. c, Percentage of migrating cells in pial side. Cells whose soma changed position within 30 min were counted as migrating cells. Values were calculated for each slice, and the average and SD for slices treated with vehicle or JNK inhibitor are shown graphically. ***p < 0.001.
Similar articles
- DLK induces developmental neuronal degeneration via selective regulation of proapoptotic JNK activity.
Ghosh AS, Wang B, Pozniak CD, Chen M, Watts RJ, Lewcock JW. Ghosh AS, et al. J Cell Biol. 2011 Sep 5;194(5):751-64. doi: 10.1083/jcb.201103153. J Cell Biol. 2011. PMID: 21893599 Free PMC article. - Role of dual leucine zipper-bearing kinase (DLK/MUK/ZPK) in axonal growth.
Eto K, Kawauchi T, Osawa M, Tabata H, Nakajima K. Eto K, et al. Neurosci Res. 2010 Jan;66(1):37-45. doi: 10.1016/j.neures.2009.09.1708. Epub 2009 Oct 4. Neurosci Res. 2010. PMID: 19808064 - Axon formation in neocortical neurons depends on stage-specific regulation of microtubule stability by the dual leucine zipper kinase-c-Jun N-terminal kinase pathway.
Hirai S, Banba Y, Satake T, Ohno S. Hirai S, et al. J Neurosci. 2011 Apr 27;31(17):6468-80. doi: 10.1523/JNEUROSCI.5038-10.2011. J Neurosci. 2011. PMID: 21525288 Free PMC article. - [Regulation of the JNK signaling pathway by dual leucine zipper kinase DLK.].
Ma XJ, Xue L. Ma XJ, et al. Yi Chuan. 2010 Aug;32(8):785-90. doi: 10.3724/sp.j.1005.2010.00785. Yi Chuan. 2010. PMID: 20709675 Review. Chinese. - From JNK to pay dirt: jun kinases, their biochemistry, physiology and clinical importance.
Karin M, Gallagher E. Karin M, et al. IUBMB Life. 2005 Apr-May;57(4-5):283-95. doi: 10.1080/15216540500097111. IUBMB Life. 2005. PMID: 16036612 Review.
Cited by
- Regulation of the Activity of the Dual Leucine Zipper Kinase by Distinct Mechanisms.
Köster KA, Dethlefs M, Duque Escobar J, Oetjen E. Köster KA, et al. Cells. 2024 Feb 11;13(4):333. doi: 10.3390/cells13040333. Cells. 2024. PMID: 38391946 Free PMC article. Review. - DLK signaling in axotomized neurons triggers complement activation and loss of upstream synapses.
Asghari Adib E, Shadrach JL, Reilly-Jankowiak L, Dwivedi MK, Rogers AE, Shahzad S, Passino R, Giger RJ, Pierchala BA, Collins CA. Asghari Adib E, et al. Cell Rep. 2024 Feb 27;43(2):113801. doi: 10.1016/j.celrep.2024.113801. Epub 2024 Feb 14. Cell Rep. 2024. PMID: 38363678 Free PMC article. - In toto imaging of glial JNK signaling during larval zebrafish spinal cord regeneration.
Becker CJ, Cigliola V, Gillotay P, Rich A, De Simone A, Han Y, Di Talia S, Poss KD. Becker CJ, et al. Development. 2023 Dec 15;150(24):dev202076. doi: 10.1242/dev.202076. Epub 2023 Dec 11. Development. 2023. PMID: 37997694 - Increase of c-FOS promoter transcriptional activity by the dual leucine zipper kinase.
Köster KA, Duque Escobar J, Fietkau A, Toledo R, Oetjen E. Köster KA, et al. Naunyn Schmiedebergs Arch Pharmacol. 2023 Jun;396(6):1223-1233. doi: 10.1007/s00210-023-02401-z. Epub 2023 Jan 26. Naunyn Schmiedebergs Arch Pharmacol. 2023. PMID: 36700987 Free PMC article. - Promoting regeneration while blocking cell death preserves motor neuron function in a model of ALS.
Wlaschin JJ, Donahue C, Gluski J, Osborne JF, Ramos LM, Silberberg H, Le Pichon CE. Wlaschin JJ, et al. Brain. 2023 May 2;146(5):2016-2028. doi: 10.1093/brain/awac415. Brain. 2023. PMID: 36342754 Free PMC article.
References
- Assadi AH, Zhang G, Beffert U, McNeil RS, Renfro AL, Niu S, Quattrocchi CC, Antalffy BA, Sheldon M, Armstrong DD, Wynshaw-Boris A, Herz J, D'Arcangelo G, Clark GD. Interaction of reelin signaling and Lis1 in brain development. Nat Genet. 2003;35:270–276. - PubMed
- Björkblom B, Östman N, Hongisto V, Komarovski V, Filén J-J, Nyman TA, Kallunki T, Courtney MJ, Coffey ET. Constitutively active cytoplasmic c-Jun N-terminal kinase 1 is a dominant regulator of dendritic architecture: role of microtubule-associated protein 2 as an effector. J Neurosci. 2005;25:6350–6351. - PMC - PubMed
- Caspi M, Atlas R, Kantor A, Sapir T, Reiner O. Interaction between LIS1 and doublecortin, two lissencephaly gene products. Hum Mol Genet. 2000;9:2205–2213. - PubMed
- Chang L, Jones Y, Ellisman MH, Goldstein LSB, Karin M. JNK1 is required for maintenance of neuronal microtubules and controls phosphorylation of microtubule-associated proteins. Dev Cell. 2003;4:521–533. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous