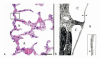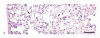Lesions of ultrasound-induced lung hemorrhage are not consistent with thermal injury - PubMed (original) (raw)
Comparative Study
Lesions of ultrasound-induced lung hemorrhage are not consistent with thermal injury
James F Zachary et al. Ultrasound Med Biol. 2006 Nov.
Abstract
Thermal injury, a potential mechanism of ultrasound-induced lung hemorrhage, was studied by comparing lesions induced by an infrared laser (a tissue-heating source) with those induced by pulsed ultrasound. A 600-mW continuous-wave CO2 laser (wavelength approximately 10.6 microm) was focused (680-microm beamwidth) on the surface of the lungs of rats for a duration between 10 to 40 s; ultrasound beamwidths were between 310 and 930 microm. After exposure, lungs were examined grossly and then processed for microscopic evaluation. Grossly, lesions induced by laser were somewhat similar to those induced by ultrasound; however, microscopically, they were dissimilar. Grossly, lesions were oval, red to dark red and extended into subjacent tissue to form a cone. The surface was elevated, but the center of the laser-induced lesions was often depressed. Microscopically, the laser-induced injury consisted of coagulation of tissue, cells and fluids, whereas injury induced by ultrasound consisted solely of alveolar hemorrhage. These results suggest that ultrasound-induced lung injury is most likely not caused by a thermal mechanism.
Figures
Fig. 1
Rat visceral pleura and the air–blood barrier. (a) The visceral pleural surface (P) of the rat lung is approximately 5 to 10 μm in thickness. It contains numerous capillaries (arrow), which separate it from the underlying air-filled alveolus (A). The box outlines the area of tissue demonstrated in Fig. 1b. Hematoxylin and eosin stain. Scale bar = 25 μm. (b) The illustration shows the area outlined by the box in Fig. 1a. The visceral pleural surface (P) of the rat lung is covered by a thin flat layer of mesothelial cells, is supported by fibrocytes and connective tissue and contains abundant capillaries (C). The air–blood barrier, formed by the endothelium of the capillary, basement membrane and type I pneumocytes (between the two arrows [this barrier varies in thickness, but has an arithmetic mean thickness of 1.25 μm, (Weibel and Knight (1964)]), is a point of impedance mismatch between the sound as it travels through tissue and fluids (blood) and abruptly hits the air in the alveolus (A). Inset: Higher magnification of the air–blood barrier (arrow head = endothelium). Transmission electron micrograph, lead citrate and uranyl acetate stain. Transmission electron micrograph courtesy of Dr. W. Haschek, Department of Pathobiology, University of Illinois.
Fig. 2
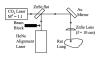
Schematic diagram of the laser irradiation arrangement.
Fig. 3
Constant temperature increase profiles of the laser irradiation of 65, 33 and 6.5° C, assuming that the temperature increase at the origin is 130° C. The laser beam's diameter(2wo) is 680 μm.
Fig. 4
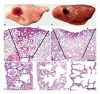
Ultrasound- and laser energy-induced lesions in rat lung. Gross lessons: (a) Ultrasound-induced lesions were consistent with hemorrhage, occurred under the visceral pleural and were oval and red to dark-red. (b) Laser energy-induced lesions were consistent with necrosis. Necrosis occurred through and under the visceral pleural, was oval and dark-red to brown. The pleural surface was injured as shown by the irregular depressed cavity in the center of the lesion. Microscopic (subgross) lesions: (c) With ultrasound, the lesion formed a “cone” of varied depths (see black lines), whose base was at the visceral pleural surface, the pleural surface was intact and elevated (hemorrhage) and the underlying alveoli were filled with hemorrhage (arrowhead = sample point for Fig. 4e). (d) With laser energy, the lesion also formed a “cone” of varied depths (see black lines) whose base was at the visceral pleural surface, the pleural surface was depressed and damaged (necrosis) and the underlying alveoli were coagulated (necrosis) (arrowhead = sample point for Fig. 4f). Microscopic lesions: (e) With ultrasound, lung had no visible lesions other than alveolar hemorrhage; (f) With laser energy, the pleura and septa were necrotic with coagulation of proteins (acute coagulative necrosis) and nucleic acid (nuclear pyknosis); and (g) Normal rat pleura and subjacent alveoli. Hematoxylin and eosin stain. Scale bar = 200 μm for Fig. 2c, d. Scale bar = 50 μm for Fig. 2e-g.
Fig. 5
Laser energy-induced lesions in rat lung. (a) The pleura is homogeneous and light pink (tissue stains poorly with hematoxylin and eosin [H&E] stain) with retention of tissue architecture (necrosis). It also contains dead cells with clumped chromatin (nuclear pyknosis). This histopathologic appearance is also present in alveolar septa and there is some cellular debris in alveoli. Red blood cells in capillaries under the pleura and in alveolar septa are necrotic and their remnants form clear oval spaces (arrow). (b) At the margins of the necrotic tissue (conical shape) deep within the lung are intact (living) alveolar septa (tissue to the right of the line formed by the arrowheads) that contain abundant erythrocytes (active hyperemia). Alveolar septa with their red blood cells (arrows) are necrotic (tissue to the left of the line formed by the arrow-heads). H&E stain. Scale bar = 50 μm.
Fig. 6
A simplified in vitro model for the region near the lung pleural surface is a water–air interface. The 6-cm diameter 3.32-MHz f/2.25 focused ultrasound source has a free-field pulse-echo −6-dB beam width at the focus of 6.7 mm. The focus is at the water–air interface. The free-field in vitro peak rarefactional pressures at the focus are 1.4 (top) and 1.8 (bottom) MPa. Their respective temporal-average intensities at the focus are 1.1 and 1.8 W/cm2. The pulse duration is 12.4 μs and the pulse repetition frequency is 1 kHz.
Similar articles
- Lung lesions induced by continuous- and pulsed-wave (diagnostic) ultrasound in mice, rabbits, and pigs.
Zachary JF, O'Brien WD Jr. Zachary JF, et al. Vet Pathol. 1995 Jan;32(1):43-54. doi: 10.1177/030098589503200106. Vet Pathol. 1995. PMID: 7725597 - Superthreshold behavior and threshold estimates of ultrasound-induced lung hemorrhage in adult rats: role of beamwidth.
O'Brien WD Jr, Simpson DG, Frizzell LA, Zachary JF. O'Brien WD Jr, et al. IEEE Trans Ultrason Ferroelectr Freq Control. 2001 Nov;48(6):1695-705. doi: 10.1109/58.971723. IEEE Trans Ultrason Ferroelectr Freq Control. 2001. PMID: 11800133 - Superthreshold behavior and threshold estimation of ultrasound-induced lung hemorrhage in adult mice and rats.
Zachary JF, Sempsrott JM, Frizzell LA, Simpson DG, O'Brien WD Jr. Zachary JF, et al. IEEE Trans Ultrason Ferroelectr Freq Control. 2001 Mar;48(2):581-92. doi: 10.1109/58.911741. IEEE Trans Ultrason Ferroelectr Freq Control. 2001. PMID: 11370372 - Section 3--selected biological properties of tissues: potential determinants of susceptibility to ultrasound-induced bioeffects. American Institute of Ultrasound in Medicine.
[No authors listed] [No authors listed] J Ultrasound Med. 2000 Feb;19(2):85-96, 154-68. doi: 10.7863/jum.2000.19.2.85. J Ultrasound Med. 2000. PMID: 10680615 Free PMC article. Review. - [Lung hemorrhage caused by diagnostic ultrasound. Review of the literature].
Rott HD. Rott HD. Ultraschall Med. 1997 Oct;18(5):226-8. doi: 10.1055/s-2007-1000430. Ultraschall Med. 1997. PMID: 9441391 Review. German.
Cited by
- Pulmonary Capillary Hemorrhage Induced by Fixed-Beam Pulsed Ultrasound.
Miller DL, Dou C, Raghavendran K. Miller DL, et al. Ultrasound Med Biol. 2015 Aug;41(8):2212-9. doi: 10.1016/j.ultrasmedbio.2015.03.030. Epub 2015 Apr 29. Ultrasound Med Biol. 2015. PMID: 25933710 Free PMC article. - A quantitative method for measuring the changes of lung surface wave speed for assessing disease progression of interstitial lung disease.
Zhang X, Zhou B, Bartholmai B, Kalra S, Osborn T. Zhang X, et al. Ultrasound Med Biol. 2019 Mar;45(3):741-748. doi: 10.1016/j.ultrasmedbio.2018.11.003. Epub 2018 Dec 29. Ultrasound Med Biol. 2019. PMID: 30598191 Free PMC article. - Ultrasound-biophysics mechanisms.
O'Brien WD Jr. O'Brien WD Jr. Prog Biophys Mol Biol. 2007 Jan-Apr;93(1-3):212-55. doi: 10.1016/j.pbiomolbio.2006.07.010. Epub 2006 Aug 8. Prog Biophys Mol Biol. 2007. PMID: 16934858 Free PMC article. Review. - An Ultrasound Surface Wave Technique for Assessing Skin and Lung Diseases.
Zhang X, Zhou B, Kalra S, Bartholmai B, Greenleaf J, Osborn T. Zhang X, et al. Ultrasound Med Biol. 2018 Feb;44(2):321-331. doi: 10.1016/j.ultrasmedbio.2017.10.010. Epub 2017 Dec 1. Ultrasound Med Biol. 2018. PMID: 29195756 Free PMC article. - The angiogenic response is dependent on ultrasound contrast agent concentration.
Johnson CA, O'Brien WD Jr. Johnson CA, et al. Vasc Cell. 2012 May 15;4:10. doi: 10.1186/2045-824X-4-10. Vasc Cell. 2012. PMID: 22587914 Free PMC article.
References
- AIUM Mechanical Bioeffects from Diagnostic Ultrasound: AIUM Consensus Statements, Section 6—Mechanical bioeffects in the presence of gas-carrier ultrasound contrast agents. American Institute of Ultrasound in Medicine [review] J Ultrasound Med. 2000b Feb;19(2):120–142. 154–168, 2000b. - PMC - PubMed
- AIUM/NEMA . Acoustic Output Measurement Standard for Diagnostic Ultrasound Equipment. American Institute of Ultrasound in Medicine; Laurel, MD: 1998. and National Electrical Manufacturers Association, Rosslyn, VA.
- Bauld TJ, Schwan HP. Attenuation and reflection of ultrasound in canine lung tissue. J Acoust Soc Am. 1974;56(5):1630–1637. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical