Threshold estimation of ultrasound-induced lung hemorrhage in adult rabbits and comparison of thresholds in mice, rats, rabbits and pigs - PubMed (original) (raw)
Comparative Study
Threshold estimation of ultrasound-induced lung hemorrhage in adult rabbits and comparison of thresholds in mice, rats, rabbits and pigs
William D O'Brien Jr et al. Ultrasound Med Biol. 2006 Nov.
Abstract
The objective of this study was to assess the threshold and superthreshold behavior of ultrasound (US)-induced lung hemorrhage in adult rabbits to gain greater understanding about species dependency. A total of 99 76 +/- 7.6-d-old 2.4 +/- 0.14-kg New Zealand White rabbits were used. Exposure conditions were 5.6-MHz, 10-s exposure duration, 1-kHz PRF and 1.1-micros pulse duration. The in situ (at the pleural surface) peak rarefactional pressure, p(r(in situ)), ranged between 1.5 and 8.4 MPa, with nine acoustic US exposure groups plus a sham exposure group. Rabbits were assigned randomly to the 10 groups, each with 10 rabbits, except for one group that had nine rabbits. Rabbits were exposed bilaterally with the order of exposure (left then right lung, or right then left lung) and acoustic pressure both randomized. Individuals involved in animal handling, exposure and lesion scoring were blinded to the exposure condition. Probit regression analysis was used to examine the dependence of the lesion occurrence on in situ peak rarefactional pressure and order of exposure (first vs. second). Likewise, lesion depth and lesion root surface area were analyzed using Gaussian tobit regression analysis. Neither probability of a lesion nor lesion size measurements was found to be statistically dependent on the order of exposure after the effect of p(r(in situ)) was considered. Also, a significant correlation was not detected between the two exposed lung sides on the same rabbit in either lesion occurrence or size measures. The p(r(in situ)) threshold estimates (in MPa) were similar to each other across occurrence (3.54 +/- 0.78), depth (3.36 +/- 0.73) and surface area (3.43 +/- 0.77) of lesions. Using the same experimental techniques and statistical approach, great consistency of thresholds was demonstrated across three species (mouse, rat and rabbit). Further, there were no differences in the biologic mechanism of injury induced by US and US-induced lesions were similar in morphology in all species and age groups studied. The extent of US-induced lung damage and the ability of the lung to heal led to the conclusion that, although US can produce lung damage at clinical levels, the degree of damage does not appear to be a significant medical problem.
Figures
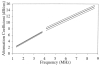
Fig. 1
Attenuation coefficient regression functions (linear regression lines ± 95% confidence band for the regression line) as a function of frequency for the intercostal tissues of New Zealand White rabbits at 37°C. The lower frequency attenuation coefficient data were derived from 16 rabbit chest walls and the higher frequency data were derived from 10 rabbit chest walls.
Fig. 2
(a) Lesion occurrence, (b) Lesion depth and (c) Lesion surface area as a function of the in situ peak rarefactional pressure. The dashed lines are straight lines connecting the mean values and are intended to provide graphical guidance. Error bars are the standard errors of the mean (n = 20 for each exposure condition except 6.65 MPa, for which n = 18).
Fig. 3
Plots of 95% confidence intervals for the estimated ED05s (solid horizontal lines with the ED05 estimates located at the centers) and ED50s (dotted horizontal lines with the ED50 estimates located at the centers) based on (a) lesion occurrence, (b) lesion depth and (c) lesion area in rabbits, rats and mice. The x-axis indicates the in situ peak rarefactional pressure, and the y-axis displays the species and study name. The two vertical lines in each box represent the weighted averages of the ED05 and ED50 estimates, respectively, with the average values at the base of each vertical line. For each of the four studies, a common exposure regime was used: 5.6-MHz center frequency, 1-kHz PRF, 10-s ED and 510-μm beamwidth.
Fig. 4
Summary of ED05 occurrence thresholds in terms of pr(in situ) (MPa) (bars; left axis) and MI (lines, right axis). * denotes the studies that are evaluated in Tables 2, 3 and 4, and Fig. 3. Error bars are SEMs. These data, by groupings from left to right, are derived, respectively, from Zachary et al. (2001a), O'Brien et al. (2001b), O'Brien et al. (2003b), O'Brien et al. (2003a), O'Brien et al. (2003a), and this study.
Fig. 5
Summary of occurrence thresholds in terms of pr(in situ) (MPa) (bars; left axis) and MI (lines, right axis) from studies not using our experimental techniques and statistical approach. These data, by groupings from left to right, are estimated, respectively, from Child et al. (1990), Raeman et al. (1993), Frizzell et al. (1994), Raeman et al. (1996), Holland et al. (1996), Baggs et al. (1996), Dalecki et al. (1997a) and Dalecki et al. (1997b).
Fig. 6
Global summary of occurrence thresholds in terms of in situ peak rarefactional pressure as a function of frequency for four species (see Figs. 4 and 5) The solid line is the threshold equation derived from pre-2000 data (AIUM 2000).
Fig. 7
Global summary of occurrence thresholds in terms of the MI as a function of frequency for four species (see Figs. 4 and 5). The solid line denotes an MI of 1.9, the FDA regulatory limit (FDA 1997).
Similar articles
- Superthreshold behavior and threshold estimation of ultrasound-induced lung hemorrhage in pigs: role of age dependency.
O'Brien WD Jr, Simpson DG, Ho MH, Miller RJ, Frizzell LA, Zachary JF. O'Brien WD Jr, et al. IEEE Trans Ultrason Ferroelectr Freq Control. 2003 Feb;50(2):153-69. doi: 10.1109/tuffc.2003.1182119. IEEE Trans Ultrason Ferroelectr Freq Control. 2003. PMID: 12625588 - Threshold estimates and superthreshold behavior of ultrasound-induced lung hemorrhage in adult rats: role of pulse duration.
O'Brien WD, Simpson DG, Frizzell LA, Zachary JF. O'Brien WD, et al. Ultrasound Med Biol. 2003 Nov;29(11):1625-34. doi: 10.1016/j.ultrasmedbio.2003.08.002. Ultrasound Med Biol. 2003. PMID: 14654157 - Superthreshold behavior and threshold estimation of ultrasound-induced lung hemorrhage in adult mice and rats.
Zachary JF, Sempsrott JM, Frizzell LA, Simpson DG, O'Brien WD Jr. Zachary JF, et al. IEEE Trans Ultrason Ferroelectr Freq Control. 2001 Mar;48(2):581-92. doi: 10.1109/58.911741. IEEE Trans Ultrason Ferroelectr Freq Control. 2001. PMID: 11370372 - Effects of pulsed ultrasound on the mouse neonate: hind limb paralysis and lung hemorrhage.
Frizzell LA, Chen E, Lee C. Frizzell LA, et al. Ultrasound Med Biol. 1994;20(1):53-63. doi: 10.1016/0301-5629(94)90017-5. Ultrasound Med Biol. 1994. PMID: 8197627 Review. - Section 4--bioeffects in tissues with gas bodies. American Institute of Ultrasound in Medicine.
[No authors listed] [No authors listed] J Ultrasound Med. 2000 Feb;19(2):97-108, 154-68. doi: 10.7863/jum.2000.19.2.97. J Ultrasound Med. 2000. PMID: 10680616 Free PMC article. Review.
Cited by
- Acoustic radiation force impulse-induced lung hemorrhage: investigating the relationship with peak rarefactional pressure amplitude and mechanical index in rabbits.
Takayama N, Sasanuma H, Rifu K, Nitta N, Akiyama I, Taniguchi N. Takayama N, et al. J Med Ultrason (2001). 2023 Apr;50(2):143-150. doi: 10.1007/s10396-023-01285-z. Epub 2023 Feb 11. J Med Ultrason (2001). 2023. PMID: 36773104 Free PMC article. - The Impact of Hemorrhagic Shock on Lung Ultrasound-Induced Pulmonary Capillary Hemorrhage.
Miller DL, Dou C, Raghavendran K, Dong Z. Miller DL, et al. J Ultrasound Med. 2021 Apr;40(4):787-794. doi: 10.1002/jum.15463. Epub 2020 Aug 28. J Ultrasound Med. 2021. PMID: 32856724 Free PMC article. - Extracorporeal Shock Waves Increase Markers of Cellular Proliferation in Bronchial Epithelium and in Primary Bronchial Fibroblasts of COPD Patients.
Di Stefano A, Frairia R, Ricciardolo FLM, Gnemmi I, Marino Gammazza A, Piraino A, Cappello F, Balbi B, Catalano MG. Di Stefano A, et al. Can Respir J. 2020 Aug 8;2020:1524716. doi: 10.1155/2020/1524716. eCollection 2020. Can Respir J. 2020. PMID: 32831979 Free PMC article. - Flow Augmentation in the Myocardium by Ultrasound Cavitation of Microbubbles: Role of Shear-Mediated Purinergic Signaling.
Moccetti F, Belcik T, Latifi Y, Xie A, Ozawa K, Brown E, Davidson BP, Packwood W, Ammi A, Huke S, Lindner JR. Moccetti F, et al. J Am Soc Echocardiogr. 2020 Aug;33(8):1023-1031.e2. doi: 10.1016/j.echo.2020.03.016. Epub 2020 Jun 10. J Am Soc Echocardiogr. 2020. PMID: 32532642 Free PMC article. - The safety of pulmonary ultrasonography in the neonatal intensive care unit.
Jagła M, Krzeczek O, Buczyńska A, Zakrzewska Z, Kwinta P. Jagła M, et al. Dev Period Med. 2018;22(1):75-80. doi: 10.34763/devperiodmed.20182201.7580. Dev Period Med. 2018. PMID: 29641425 Free PMC article.
References
- American Institute of Ultrasound in Medicine and National Electrical Manufacturers Association . Acoustic output measurement standard for diagnostic ultrasound equipment. Laurel, MD and Rosslyn, VA: 1998.
- American Institute of Ultrasound in Medicine and National Electrical Manufacturers Association . Standard for real-time display of thermal and mechanical acoustic output indices on diagnostic ultrasound equipment, Rev. 2. Laurel, MD and Rosslyn, VA: 2004.
- Amemiya T. Tobit models: A survey. J Econometrics. 1984;24:3–61.
- Baggs R, Penney DP, Cox C, et al. Thresholds for ultrasonically induced lung hemorrhage in neonatal swine. Ultrasound Med Biol. 1996;22:119–128. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical