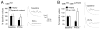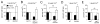Mechanisms underlying the rapid induction and sustained expression of synaptic homeostasis - PubMed (original) (raw)
Mechanisms underlying the rapid induction and sustained expression of synaptic homeostasis
C Andrew Frank et al. Neuron. 2006.
Abstract
Homeostatic signaling systems are thought to interface with the mechanisms of neural plasticity to achieve stable yet flexible neural circuitry. However, the time course, molecular design, and implementation of homeostatic signaling remain poorly defined. Here we demonstrate that a homeostatic increase in presynaptic neurotransmitter release can be induced within minutes following postsynaptic glutamate receptor blockade. The rapid induction of synaptic homeostasis is independent of new protein synthesis and does not require evoked neurotransmission, indicating that a change in the efficacy of spontaneous quantal release events is sufficient to trigger the induction of synaptic homeostasis. Finally, both the rapid induction and the sustained expression of synaptic homeostasis are blocked by mutations that disrupt the pore-forming subunit of the presynaptic Ca(V)2.1 calcium channel encoded by cacophony. These data confirm the presynaptic expression of synaptic homeostasis and implicate presynaptic Ca(V)2.1 in a homeostatic retrograde signaling system.
Figures
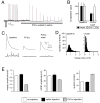
Figure 1. Rapid induction of synaptic homeostasis following injection of a glutamate receptor antagonist.
A) Sample recording from the NMJ showing a stimulus-dependent decline in mepsp and EPSP amplitudes following PhTox bath application (horizontal line). B) Average mepsp, EPSP amplitudes and quantal content (Qc) prior to PhTox application (baseline; n=12) and following a 2 min PhTox incubation with 30 sec washout (PhTox-wash; n=13). Recording saline lacks PhTox. C) Sample traces for the data presented in (B). D) Histograms reveal that the mepsp amplitude distribution is shifted toward smaller values following PhTox application and washout (right graph; wash). Samples sizes are equivalent. E) Quantification of average mepsp amplitude (graph at left), average EPSP amplitude (middle graph) and quantal content (graph at right). Quantification was performed for three conditions including: 30 min after 200μM PhTox injection (PhTox injection; n=30), 30 min after saline injection (saline injection; n=17) versus uninjected controls (no injection, n=10). There is a significant, homeostatic increase in quantal content in the PhTox-injected animals compared to controls (p<0.001).
Figure 2. Rapid induction synaptic homeostasis in a semi-intact preparation
A) Quantification of average mepsp amplitude (left), average EPSP amplitude (middle) and quantal content (right) for the three conditions listed below the graphs; 10min saline incubation (control incubation), 30 second incubation in PhTox (30sec PhTox) and a 10 minute incubation in PhTox (10min PhTox). There is a statistically significant increase in quantal content after a 10 min PhTox incubation (n=48; p<0.01) compared to a 30 sec PhTox incubation (n=10) or saline incubation (control incubation; n=42). **B)** Sample traces prior to and following PhTox incubation for 10min. Scale bar 150ms; 5mV for EPSPs and 150ms; 1mV for mepsps. **C)** Quantification as in (A). Incubating _GluRIIA_ mutants for 10 min in PhTox (n=12) does not reduce average mepsp amplitude beyond that observed in _GluRIIA_ mutants without toxin (p>0.2; n=12) and does not increase quantal content beyond that observed in GluRIIA mutants without toxin (p>0.5). D) Data are shown for wild-type synapses incubated in control saline (10 min) versus wild-type synapses incubated in NSTX-3 (NSTX-3; 10μM, 15 min). Prior to recording, synapses were washed in normal saline without NSTX-3. A persistent inhibition of postsynaptic receptors following the wash is shown by the significant decrease in mepsp amplitudes compared to mock treated wild-type controls (p<0.01). After a 15 min incubation in NSTX-3, EPSP amplitudes are near wild-type values (p>0.1) and there is a significant increase in quantal content (p<0.01).
Figure 3. Evidence for increased release probability during the rapid induction of synaptic homeostasis.
A) Quantal content, estimated by the method of failures in 0.15mM Ca2+ is significantly increased following application of PhTox (+PhTox; 10min semi-intact preparation) compared to wild type in the absence of PhTox (p<0.01). Quantal content values are: wild type (0.43 ± 0.04, N=14) and PhTox (0.68 ± 0.05, N=15). B) Sample histogram of EPSP amplitudes measured in wild type. Inset shows sample traces from this recording. Three stimuli are shown for each trace (vertical transients are stimulus artifacts). Blue stars indicate stimulus-locked release events and red stars indicated putative failures following the stimulus artifact. C) Sample histogram of EPSP amplitudes measured at a PhTox treated synapse with inset as in (B). D) Graph examining vesicle depletion during a stimulus train (6000 stimuli at 8Hz) comparing wild type (white symbols; N=6) to GluRIIA mutant (blue symbols; N=6) and PhTox treated (10 min semi-intact; N=8) animals. The average value (± SEM) is shown for each stimulus to generate the curves. There is significantly greater EPSP rundown comparing GluRIIA and PhTox treated animals with wild type (p<0.01). E) Sample traces for data shown in (D). There is significantly greater EPSP rundown in GluRIIA and PhTox treated synapses. Rapid recovery to initial EPSP amplitudes is demonstrated by low frequency stimulation (0.2Hz) following the cessation of the stimulus train.
Figure 4. Induction of synaptic homeostasis in real time.
A) mepsp, EPSP and quantal content are plotted over time for a single recording (normalized to baseline values prior to toxin application). Time zero is the time of toxin application. EPSPs delivered at 0.2Hz are averaged every 50 s. mepsps are averaged over identical 50 s intervals. Quantal content is calculated based on these average measurements for each 50 s interval. Baseline stimulation is followed by a perfusion with PhTox (4μM) that remains present in the bath solution throughout the remainder of the recording. 10 stimuli are delivered immediately following PhTox application to maximally suppress mepsp amplitudes (amplitudes not shown on graph). 0.2Hz stimulation is then resumed for the duration of the experiment in the continued presence of PhTox. B) EPSP amplitudes for the experiment in (A). C) Average values for experiments performed as in (A) without the addition of PhTox (n=10). D) Average values for all experiments that included PhTox as in (A) (n=12).
Figure 5. Motoneuron activity is not required for the rapid induction of synaptic homeostasis.
A) Quantal content is shown for individual recordings and presented as a percent relative to the average quantal content observed in wild type animals recorded in normal saline (dotted line at 100%). In each preparation recordings were made from muscle 6 in segment A3 on both sides of the animal (coded with the same color symbol and connected by a line). In one hemi-segment the motor axons were severed prior to application of PhTox (cut) and in the other hemi-segment the motor axons remained intact (uncut). Every recording showed a homeostatic increase in quantal content. There is no obvious trend in the degree of compensation comparing cut versus uncut axons in a single animal. B) Average values for the data presented in (A). The cut axons show a statistically significant increase in quantal content compared to controls (p<0.01). The quantal content recorded in uncut axons is not significantly greater than that observed in cut axons (p>0.1). C) A sample 10 minute recording showing the induction of synaptic homeostasis in the absence of evoked transmission. The recording is initiated in the absence of PhTox and 5 stimuli are delivered to quantify basal synaptic efficacy (*1). PhTox is then applied without further nerve stimulation. The absence of evoked neurotransmission is evident until test stimuli are delivered at the end of the recording (10 action potentials; *2). D) Sample EPSPs from the recording in (C) from the indicated time points. At time point (*2) the EPSP amplitude is close to baseline while mepsps are significantly smaller than baseline. (E) Average data for experiments as in (C) calculating quantal content (red), EPSP amplitude (blue) and mepsp amplitude (white). The average mepsp amplitude is calculated at 30s intervals. No evoked release was observed during PhTox incubation until test stimuli are delivered at the end of the recording. In the absence of evoked neurotransmission PhTox application (over time indicated) induces a significant, homeostatic increase in quantal content compared to baseline values prior to PhTox application (p<0.01).
Figure 6. Presynaptic release precisely offsets decreased quantal size
Data for individual recordings are shown, plotting quantal content versus mepsp amplitude. Each point is a separate experiment. (A) Wild type synapse (white) and synapses incubated in PhTox for 10 min (red) are shown. Quantal content scales continuously with decreased mepsp amplitude. The line represents the function describing perfect compensation (quantal content/mepsp = constant EPSP). The constant EPSP value is taken as the average wild-type amplitude (no drug). B) Data plotted as in (A) for wild-type (white) versus GluRIIA mutant animals.
Figure 7. The induction of synaptic homeostasis is suppressed by mutations in the CaV2.1 alpha-1 subunit
A) Average mepsp and quantal content, normalized to baseline, for cacTS2 animals with and without PhTox incubation (10 min). Sample traces are at right. No homeostatic increase in quantal content is observed following PhTox incubation (p>0.4; n=13). B) Quantification as in (A) for the cacTS2cacsu(TS2)2 intragenic suppressor double mutation. Sample traces at right, scale as in (A). A homeostatic increase in quantal content is restored in the cacTS2cacsu(TS2)2 double mutant (p<0.02; n=13).
Figure 8. The expression of synaptic homeostasis is suppressed by mutations in the CaV2.1 alpha-1 subunit
A) Quantification of average mepsp amplitude (filled bars) and quantal content (open bars). Values are normalized to wild-type control (dotted line). GluRIIA mutants have decreased mepsp amplitudes and a homeostatic increase in quantal content (p<0.001 for both). **B**) Values as in (A) are normalized to those observed in the _cacS_/+ heterozygous condition. A homeostatic increase in quantal content is observed in the _cacS_/+; _GluRIIA_ animals (p<0.01). However, the magnitude of the increase in quantal content is significantly less than that observed in _GluRIIA_ mutants shown in (A) (p<0.01). **C**) Quantification as in (A) for control and _cacS;GluRIIA_ double mutants. Data are normalized to values observed in _cacS_ alone. A homeostatic increase in quantal content is suppressed in the _cacS;GluRIIA_ double mutant compared to _cacS_ alone (p>0.2). D) Quantification as in (A) for control and GluRIIA mutants recorded in 1.5mM extracellular calcium. GluRIIA mutants show a compensatory increase in quantal content at 1.5mM extracellular calcium compared to wild type (p<0.01). Quantal contents were corrected for non-linear summation prior to normalization. **E**) The homeostatic increase in quantal content is suppressed in the _cacS;GluRIIA_ double mutant compared to _cacS_ mutants alone, recorded in 1.5mM extracellular calcium (p>0.5).
Figure 9. Conditional disruption of synaptic homeostasis using temperature sensitive mutations in the CaV2.1 alpha-1 subunit.
A) Quantification of average mepsp amplitude (filled bars) and quantal content (open bars). Values are normalized to wild-type control (dotted line) recorded in 0.5mM extracellular calcium. (Left) GluRIIA mutants have decreased mepsp amplitudes and a homeostatic increase in quantal content (p<0.001 for both). Data are re-plotted from Figure 8A for figure clarity. (Right) There remains a significant homeostatic increase in quantal content in the _cacTS2; GluRIIA_ double mutant compared to _cacTS2_ alone (p<0.01). **B**) Quantification as in (A) for wild type (wt), _GluRIIA, cacTS2_ and _cacTS2;GluRIIA_ raised at 29°C (assayed at room temperature, 0.5mM Ca2+). (Left) _GluRIIA_ animals show a compensatory increase in quantal content compared to wild type (p<0.01). At right, the _cacTS2;GluRIIA_ double mutants fail to show a homeostatic increase in quantal content when compared to _cacTS2_ alone (p>0.25). C) Quantification as in (A) for the double mutant cacTS2, cacsu(TS2)2 and the triple mutant cacTS2, cacsu(TS2)2;GluRIIA raised at 29°C (assayed at room temperature, 0.5mM Ca2+). Values are normalized to cacTS2, cacsu(TS2)2. A homeostatic increase in quantal content is observed in triple mutant animals (p<0.01) indicating partial restoration of homeostatic compensation by the intragenic suppressor mutation. **D**) Quantification as in (A) for wild type (wt), _GluRIIA, cacTS2_ and _cacTS2;GluRIIA_ for the indicated calcium and rearing conditions. Data for wt and _GluRIIA_ are repeated from figure 8D for visual clarity. At right, the _cacTS2;GluRIIA_ double mutants fail to show a significant increase in quantal content compared to _cacTS2_ alone (p>0.25). Quantal contents were corrected for non-linear summation prior to normalization. E) cacTS2 and cacTS2;GluRIIA animals were raised at room temperature for three days of larval development before being shifted to 29°C for the final 24 hours of larval development. A homeostatic increase in quantal content is observed (p=0.03). However, the homeostatic increase in quantal content is significantly suppressed (p<0.01) in the _cacTS2; GluRIIA_ double mutant animals compared to that observed when _cacTS2;GluRIIA_ animals are raised at 22°C throughout development (A – right graph). Furthermore, the increase in quantal content is not different from that observed when _cacTS2;GluRIIA_ animals are raised at 29°C throughout development (B – right graph). Throughout, the notation ‘ns’ indicates that quantal content is not significantly different (p>0.25). Sample size for each genotype and non-normalized values (including EPSP amplitudes) are presented in Table 1.
Comment in
- Synaptic homeostasis on the fast track.
Macleod GT, Zinsmaier KE. Macleod GT, et al. Neuron. 2006 Nov 22;52(4):569-71. doi: 10.1016/j.neuron.2006.11.006. Neuron. 2006. PMID: 17114040 Review.
Similar articles
- α2δ-3 Is Required for Rapid Transsynaptic Homeostatic Signaling.
Wang T, Jones RT, Whippen JM, Davis GW. Wang T, et al. Cell Rep. 2016 Sep 13;16(11):2875-2888. doi: 10.1016/j.celrep.2016.08.030. Cell Rep. 2016. PMID: 27626659 Free PMC article. - Homeostatic synaptic depression is achieved through a regulated decrease in presynaptic calcium channel abundance.
Gaviño MA, Ford KJ, Archila S, Davis GW. Gaviño MA, et al. Elife. 2015 Apr 17;4:e05473. doi: 10.7554/eLife.05473. Elife. 2015. PMID: 25884248 Free PMC article. - The Drosophila cacts2 mutation reduces presynaptic Ca2+ entry and defines an important element in Cav2.1 channel inactivation.
Macleod GT, Chen L, Karunanithi S, Peloquin JB, Atwood HL, McRory JE, Zamponi GW, Charlton MP. Macleod GT, et al. Eur J Neurosci. 2006 Jun;23(12):3230-44. doi: 10.1111/j.1460-9568.2006.04873.x. Eur J Neurosci. 2006. PMID: 16820014 - Synaptic homeostasis on the fast track.
Macleod GT, Zinsmaier KE. Macleod GT, et al. Neuron. 2006 Nov 22;52(4):569-71. doi: 10.1016/j.neuron.2006.11.006. Neuron. 2006. PMID: 17114040 Review. - Synaptic vesicle pools and plasticity of synaptic transmission at the Drosophila synapse.
Kidokoro Y, Kuromi H, Delgado R, Maureira C, Oliva C, Labarca P. Kidokoro Y, et al. Brain Res Brain Res Rev. 2004 Dec;47(1-3):18-32. doi: 10.1016/j.brainresrev.2004.05.004. Brain Res Brain Res Rev. 2004. PMID: 15572160 Review.
Cited by
- Plasticity in respiratory motor neurons in response to reduced synaptic inputs: A form of homeostatic plasticity in respiratory control?
Braegelmann KM, Streeter KA, Fields DP, Baker TL. Braegelmann KM, et al. Exp Neurol. 2017 Jan;287(Pt 2):225-234. doi: 10.1016/j.expneurol.2016.07.012. Epub 2016 Jul 22. Exp Neurol. 2017. PMID: 27456270 Free PMC article. Review. - Spontaneous transmitter release is critical for the induction of long-term and intermediate-term facilitation in Aplysia.
Jin I, Puthanveettil S, Udo H, Karl K, Kandel ER, Hawkins RD. Jin I, et al. Proc Natl Acad Sci U S A. 2012 Jun 5;109(23):9131-6. doi: 10.1073/pnas.1206914109. Epub 2012 May 22. Proc Natl Acad Sci U S A. 2012. PMID: 22619320 Free PMC article. - GABAB receptor deficiency causes failure of neuronal homeostasis in hippocampal networks.
Vertkin I, Styr B, Slomowitz E, Ofir N, Shapira I, Berner D, Fedorova T, Laviv T, Barak-Broner N, Greitzer-Antes D, Gassmann M, Bettler B, Lotan I, Slutsky I. Vertkin I, et al. Proc Natl Acad Sci U S A. 2015 Jun 23;112(25):E3291-9. doi: 10.1073/pnas.1424810112. Epub 2015 Jun 8. Proc Natl Acad Sci U S A. 2015. PMID: 26056260 Free PMC article. - Down-Regulation of Double C2 Domain Alpha Promotes the Formation of Hyperplastic Nerve Fibers in Aganglionic Segments of Hirschsprung's Disease.
Xiao J, Meng X, Chen K, Wang J, Wu L, Chen Y, Yu X, Feng J, Li Z. Xiao J, et al. Int J Mol Sci. 2022 Sep 6;23(18):10204. doi: 10.3390/ijms231810204. Int J Mol Sci. 2022. PMID: 36142117 Free PMC article. - Spontaneously Recycling Synaptic Vesicles Constitute Readily Releasable Vesicles in Intact Neuromuscular Synapses.
Egashira Y, Kumade A, Ojida A, Ono F. Egashira Y, et al. J Neurosci. 2022 Apr 27;42(17):3523-3536. doi: 10.1523/JNEUROSCI.2005-21.2022. Epub 2022 Mar 24. J Neurosci. 2022. PMID: 35332083 Free PMC article.
References
- Atwood HL, Govind CK, Wu CF. Differential ultrastructure of synaptic terminals on ventral longitudinal abdominal muscles in Drosophila larvae. J Neurobiol. 1993;24:1008–1024. - PubMed
- Barrett CF, Cao YQ, Tsien RW. Gating deficiency in a familial hemiplegic migraine type 1 mutant P/Q-type calcium channel. J Biol Chem. 2005;280:24064–24071. - PubMed
- Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC. Control of synaptic strength by glial TNFalpha. Science. 2002;295:2282–2285. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS039313/NS/NINDS NIH HHS/United States
- F32 NS049694-03/NS/NINDS NIH HHS/United States
- NS049694/NS/NINDS NIH HHS/United States
- R01 NS039313-07/NS/NINDS NIH HHS/United States
- F32 NS049694/NS/NINDS NIH HHS/United States
- NS39313/NS/NINDS NIH HHS/United States
- F32 NS049694-02/NS/NINDS NIH HHS/United States
- F32 NS049694-01/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases