Molecular markers of neuronal progenitors in the embryonic cerebellar anlage - PubMed (original) (raw)
Comparative Study
Molecular markers of neuronal progenitors in the embryonic cerebellar anlage
Daniver Morales et al. J Neurosci. 2006.
Abstract
The cerebellum, like the cerebrum, includes a nuclear structure and an overlying cortical structure. Experiments in the past decade have expanded knowledge beyond the traditional function of the cerebellum to include critical roles in motor learning and memory and sensory discrimination. The initial steps in cerebellar development depend on inductive signaling involving FGF and Wnt proteins produced at the mesencephalic/metencephalic boundary. To address the issue of how individual cerebellar cell fates within the cerebellar territory are specified, we examined the expression of transcription factors, including mammalian homologues of LIM homeodomain-containing proteins, basic helix-loop-helix proteins, and three amino acid loop-containing proteins. The results of these studies show that combinatorial codes of transcription factors define precursors of the cerebellar nuclei, and both Purkinje cells and granule neurons of the cerebellar cortex. Examination of gene expression patterns in several hundred lines of Egfp-BAC (bacterial artificial chromosome) transgenic mice in the GENSAT Project revealed numerous genes with restricted expression in cerebellar progenitor populations, including genes specific for cerebellar nuclear precursors and Purkinje cell precursors. In addition, we identified patterns of gene expression that link granule and Purkinje cells to their precerebellar nuclei. These results identify molecular pathways that offer new insights on the development of the nuclear and cortical structures of the cerebellum, as well as components of the cerebellar circuitry.
Figures
Figure 1.
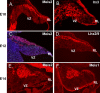
Precursors of the neurons of the cerebellar nuclei express the transcription factors IRX3, MEIS1, MEIS2, and LHX2/9. On E10.25, the initial progenitor population generated in the VZ lining the fourth ventricle gives rise to progenitors of the cerebellar nuclei. At this age, postmitotic cells are labeled with β-tubulin (data not shown). At E10.25 (A), MEIS2 cells are labeled on the surface of the developing cerebellar anlage. Irx3-positive cells are evident on the surface (B) but also in transit from the VZ to the surface. By E12.5 (C), cells that exit the cell cycle on E10.25, as indicated by BrdU labeling (C, green), are located on the surface of the emerging cerebellar anlagen. Double labeling with antibodies against MEIS2 (red) confirms the expression of the transcription factor MEIS2; the total cell population is labeled with the marker 4′,6′-diamidino-2-phenylindole (blue). D, Labeling with antibodies against LHX2/9. By E14.5, cells labeled with antibodies against MEIS2 (E) and MEIS1 (F) have migrated from the surface and begun to assemble into nascent cerebellar nuclei in the rostral aspect of the cerebellar territory.
Figure 2.
Migratory pathways of precursors of the neurons of cerebellar nuclei. At E10.25, the first precursors of the CN are migrating from the VZ to the surface of the anlagen. Double labeling with a marker for radial glia RC2 (red) and the EGFP reporter (green) on sections of _Meis2-Egfp_-BAC transgenic animals reveals a close apposition of migrating neurons to the radial glial pathway. In B, similar results are observed with Irx3+ progenitors from _Irx3-Egfp_-BAC transgenic mice (green) and RC2+ radial glia. In the lower left area of B, a high-magnification image of the cell marked with an arrow is illustrated. In C, aggregating _Irx3_+ CN cells form distinct nuclei interconnected by fibers (EGFP labeling in _Irx3-Egfp_-BAC transgenic mice detected by DAB localization). In D, MEIS2 expression is illustrated in the cerebellar nuclei (immunostaining with antibodies against MEIS2). On the right, a general schema for the formation of the cerebellar nuclei is shown in a drawing of a whole mount of the midbrain/hindbrain region between E12.5 (left side of drawing) and E15.5 (right side of drawing). The dorsal surface of the cerebellar anlage is “D,” and the ventral midline is “V.” At the left, the orange zone on the dorsal surface is occupied by precursors of cerebellar nuclei progenitors on E12.5. By E 15.5, these cells exit the surface and form three distinct nuclei (green stripes); the EGL (blue) now occupies the dorsal surface.
Figure 3.
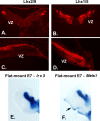
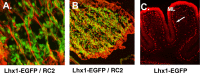
Expression of transcription factors in progenitor cell populations of the embryonic chick cerebellum. To examine whether the patterns of gene expression we observed were evident across species, we performed localization studies in embryonic chick cerebellum. In transverse sections at E4.5, antibodies against LHX2/9 label prospective cerebellar nuclear cells on the surface of the cerebellum (A), and antibodies against LHX1/5 label cells in a zone beneath the cell surface (B). In sagittal sections of E5 chick, LHX2/9+ cells are evident on the surface of the developing cerebellar anlagen (C) and LHX1/5 PC precursors beneath the superficial zone occupied by CN progenitors (D). In flat mount preparations of E7 chick (see Fig. 2 for schematic), in situ hybridization with a probe for Irx3 (E) reveals that Irx3 transcripts are restricted to the cerebellar nuclei, whereas hybridization with a probe for Meis1 (F) reveals that Meis1 transcripts are expressed in cerebellar nuclei and in the EGL on the dorsal surface at right (arrow).
Figure 4.
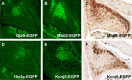
Markers for precursors of the neurons of cerebellar nuclei. Analysis of _Egfp_-BAC transgenic mice generated by the GENSAT project illustrates at least four markers for progenitors of the CN at E15.5. EGFP is expressed in cells located in the region occupied by precursors of the CN in the cerebellar territory of Gja9-Egfp BAC transgenic mice (A), _Mbd2-Egfp_-BAC transgenic mice (B, C), _Htr3a-Egfp_-BAC transgenic mice (C), and Kcnj5-Egfp BAC transgenic mice (D, E). As discussed previously, GJA9, a member of the connexin family, functions in temporary physiological connections, typical of embryonic neurons; MBD2 is thought to function in transcriptional repression; HTR3a is a serotonin receptor; and KCNJ5 is a potassium inwardly rectifying channel. A, B, D, E, Unstained sections; C, F, sections immunostained with antibodies against EGFP (DAB).
Figure 5.
Purkinje cell progenitors express LIM homeodomain transcription factors. A, At E12.5, PC progenitors that have exited the VZ express LHX1/5. In B, LHX1/5 expression continues as the number of PC progenitors increases and forms a dense zone of cells beneath the dorsal surface of the anlagen. At E14, the earliest stage at which PC progenitors express the signature PC marker CALB1 (C), EGFP+ cells are evident in sections of _Lhx1-Egfp_-BAC transgenic mice in this zone. In D, staining with antibodies against CALB1 also reveals Purkinje cell progenitors, many of which are double labeled with EGFP (E). Identical results were obtained in double-labeling experiments with cells from _Lhx5-Egfp_-BAC mice (data not shown). Thus, Lhx1 and Lhx5 are expressed in progenitors of cerebellar Purkinje cells 2 d before expression of CALB1.
Figure 6.
Purkinje cell progenitors migrate on the radial glial scaffold. Although previous studies indicate that CALB1+ cells migrate along VIMENTIN+ fibers, CALB1 is a relatively late PC marker, and VIMENTIN labels both neuronal and glial fibers. In A, at E11.5, cells expressing EGFP in _Lhx1-Egfp_-BAC-transgenic mice migrate along RC2+ radial glial fibers. In B, at lower power, large numbers of EGFP+ cells associate with immunostained radial glial fibers, indicating that PC progenitors use the radial glial pathway for their migration from the VZ out into the middle of the emerging cerebellar anlagen (E14.5). In the neonatal cerebellum (C), antibodies against LHX1/5 proteins label both Purkinje neurons (arrow) and interneurons in the molecular layer (ML).
Figure 7.
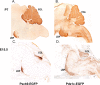
RGS8 is a marker for purkinje cell progenitors. Analysis of the Egfp-BAC transgenic mice generated in the GENSAT Project reveals expression of the RGS8 gene at E15.5 in cells thought to be precursors of Purkinje cells (A). Fluorescence imaging of sections of _Rgs8-Egfp_-BAC transgenic mouse cerebellum at P7 reveals bright labeling of immature Purkinje cells (B), their dendrites, and their axonal projections (arrows). In C, localization of the EGFP reporter gene in the cerebellar territory of _Pcp2-Egfp_-BAC transgenic mice indicates robust EGFP expression in the zone occupied by immature Purkinje cells at this stage. D indicates the restricted expression of the EGFP reporter gene for _Pcp2-Egfp_-BAC transgenic animals at E15.5, because cells other than PC progenitors in the emerging cerebellar territory are unlabeled. CP, Choroid plexus; S, surface.
Figure 8.
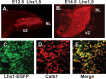
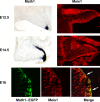
Meis1 expression by precursors of the CN and a subpopulation of EGL cells, which generate cortical granule neurons. Top, At E12.5, Math1 expression is confined to the anterior RL, visible as the labeled, blue structure at the bottom right of the tissue (left). Antibodies against MEIS1 label precursors of the CN present on the surface of the anlagen and cells in the RL (right). Middle, By E14.5, _Math1_+ cells have spread across the surface of the anlage (left), and antibody staining reveals MEIS1 protein in the precursor cells, which are forming the CN, visible as a large round domain of positive cells just beneath the surface in the medial aspect of the cerebellar anlagen and the emerging EGL, which covers most of the surface (right). Bottom, Immunostaining of the EGFP reporter in sections of _Math1-Egfp_-BAC transgenic animals to visualize EGFP illustrates a subpopulation of cells that are double labeled with antibodies against MEIS1 (arrows) in the EGL.
Figure 9.
Genes expressed in the cerebellar EGL and in neurons of the lateral pontine nucleus. In the neonatal period, several genes are expressed in both the EGL, which will generate cortical granule neurons, and in neurons of the precerebellar nuclei in the brainstem, which form connections with granule cells. In A, the EGFP reporter illustrates strong expression of the Pcsk9 gene in EGL cortical cells and in cells of the lateral basilar pons (LBP), a structure previously shown to be formed by aRL cells, which migrate ventrally (Wingate and Hatten, 1999). More importantly, the LBP projects mossy fibers to the granule cells, and the strength of the input from mossy fibers sets the level of parallel fiber output to the PCs. In the embryonic cerebellum, EGFP expression is restricted to the EGL of the cerebellar territory and the emerging LBP in the brainstem of _Pcsk9-Egfp_-BAC transgenic mice (C). Cerebellar EGL cells also express Pde1c, beginning on E10 in _Pde1c-Egfp_-BAC transgenic mice (Gong et al., 2003). At P6 (B), the EGFP reporter is abundant in cerebellar EGL cells and in cells of both the lateral basilar pons and the olivary nucleus. This suggests that both the LBP cells, which project mossy fibers that form synapses with granule cells, and olivary neurons, which project climbing fibers that synapse with the targets of granule cells (Purkinje cells), express Pde1c. At E15.5, EGFP+ cells from E15.5 _Pde1c-Egfp_-BAC transgenic mice are evident in the cerebellar EGL, precursor neurons of the CN, the LBP, the olivary nucleus, and several other migratory pathways of rostral RL cells. As reported recently (Machold and Fishell, 2005; Wang et al., 2005), similar results are seen with EGFP labeling in _Math1-Egfp_-BAC transgenic mice where EGL cells, CN cells, basilar pons cells, and cells of other brainstem “cerebellar” nuclei contain EGFP+ cells (data not shown).
Figure 10.
A model for the generation and migratory pathways of progenitors of the cerebellar nuclei. In the mouse, one population of progenitors of the neurons of the CNPs is generated in the VZ of the emerging cerebellar anlagen. Between E10 and E12.5, this progenitor population migrates radially along the glial fiber system (data not shown) to establish a superficial layer (blue). Subsequently, progenitors of the Purkinje cell (PCP) are generated in the VZ (E11–E14.5) and migrate radially to form a zone (red) beneath the CNPs. A second germinal zone, the anterior rhombic lip (yellow; RL), generates a subpopulation of precursors of neurons of the cerebellar nuclei, precerebellar nuclei (data not shown), and the granule neurons of the cerebellar cortex. At E12.5, a layer of CNPs (blue) generated in the VZ occupy the surface of the embryonic cerebellum. Between E12.5 and E14.5, several populations of RL progenitors move up onto the surface of the anlagen (yellow). These include the bulk of the progenitor population, the precursors of the granule neuron (solid yellow), and a subpopulation of cells that intercalate among the VZ-derived CNPs (yellow dots). By E14.5, the CNPs (containing both VZ and RL derived progenitors) have migrated off of the surface and formed the nascent cerebellar nuclei. By E16, the cerebellar nuclei (blue with yellow dots) settle beneath the emerging cerebellar cortex [containing the precursors of the granule neurons (EGL, yellow)], the Purkinje neuron (red), and the interneurons. Thus, a complex pattern of histogenesis and migration set forth the architecture of the murine cerebellum.
Similar articles
- Ric-8a, a guanine nucleotide exchange factor for heterotrimeric G proteins, regulates bergmann glia-basement membrane adhesion during cerebellar foliation.
Ma S, Kwon HJ, Huang Z. Ma S, et al. J Neurosci. 2012 Oct 24;32(43):14979-93. doi: 10.1523/JNEUROSCI.1282-12.2012. J Neurosci. 2012. PMID: 23100420 Free PMC article. - Math1 is expressed in temporally discrete pools of cerebellar rhombic-lip neural progenitors.
Machold R, Fishell G. Machold R, et al. Neuron. 2005 Oct 6;48(1):17-24. doi: 10.1016/j.neuron.2005.08.028. Neuron. 2005. PMID: 16202705 - Disruption of cerebellar granule cell development in the Pax6 mutant, Sey mouse.
Swanson DJ, Tong Y, Goldowitz D. Swanson DJ, et al. Brain Res Dev Brain Res. 2005 Dec 7;160(2):176-93. doi: 10.1016/j.devbrainres.2005.09.005. Epub 2005 Nov 9. Brain Res Dev Brain Res. 2005. PMID: 16289327 - Neurogenetics of the cerebellar system.
Millen KJ, Millonig JH, Wingate RJ, Alder J, Hatten ME. Millen KJ, et al. J Child Neurol. 1999 Sep;14(9):574-81; discussion 581-2. doi: 10.1177/088307389901400905. J Child Neurol. 1999. PMID: 10488902 Review. - Development and evolution of cerebellar neural circuits.
Hashimoto M, Hibi M. Hashimoto M, et al. Dev Growth Differ. 2012 Apr;54(3):373-89. doi: 10.1111/j.1440-169X.2012.01348.x. Dev Growth Differ. 2012. PMID: 22524607 Review.
Cited by
- Distinct functional domains of Dystroglycan regulate inhibitory synapse formation and maintenance in cerebellar Purkinje cells.
Jahncke JN, Schnell E, Wright KM. Jahncke JN, et al. bioRxiv [Preprint]. 2024 Aug 30:2024.08.29.610348. doi: 10.1101/2024.08.29.610348. bioRxiv. 2024. PMID: 39257744 Free PMC article. Preprint. - Human organoid model of pontocerebellar hypoplasia 2a recapitulates brain region-specific size differences.
Kagermeier T, Hauser S, Sarieva K, Laugwitz L, Groeschel S, Janzarik WG, Yentür Z, Becker K, Schöls L, Krägeloh-Mann I, Mayer S. Kagermeier T, et al. Dis Model Mech. 2024 Jul 1;17(7):dmm050740. doi: 10.1242/dmm.050740. Epub 2024 Jul 22. Dis Model Mech. 2024. PMID: 39034883 Free PMC article. - A spatial-temporal map of glutamatergic neurogenesis in the murine embryonic cerebellar nuclei uncovers a high degree of cellular heterogeneity.
Casoni F, Croci L, Marroni F, Demenego G, Marullo C, Cremona O, Codazzi F, Consalez GG. Casoni F, et al. J Anat. 2024 Oct;245(4):560-571. doi: 10.1111/joa.14107. Epub 2024 Jul 6. J Anat. 2024. PMID: 38970393 Free PMC article. - Engrailed transcription factors direct excitatory cerebellar neuron diversity and survival.
Krishnamurthy A, Lee AS, Bayin NS, Stephen DN, Nasef O, Lao Z, Joyner AL. Krishnamurthy A, et al. Development. 2024 Jul 15;151(14):dev202502. doi: 10.1242/dev.202502. Epub 2024 Jul 26. Development. 2024. PMID: 38912572 - Tools for _Cre_-Mediated Conditional Deletion of Floxed Alleles from Developing Cerebellar Purkinje Cells.
Jahncke JN, Wright KM. Jahncke JN, et al. eNeuro. 2024 Jun 3;11(6):ENEURO.0149-24.2024. doi: 10.1523/ENEURO.0149-24.2024. Print 2024 Jun. eNeuro. 2024. PMID: 38777609 Free PMC article.
References
- Alder J, Cho NK, Hatten ME. Embryonic precursor cells from the rhombic lip are specified to a cerebellar granule neuron identity. Neuron. 1996;17:389–399. - PubMed
- Altman J, Bayer SA. Embryonic development of the rat cerebellum. III. Regional differences in the time of origin, migration, and settling of Purkinje cells. J Comp Neurol. 1985;231:42–65. - PubMed
- Anthony TE, Klein C, Fishell G, Heintz N. Radial glia serve as neuronal progenitors in all regions of the central nervous system. Neuron. 2004;41:881–890. - PubMed
- Batini C. Cerebellar localization and colocalization of GABA and calcium binding protein-D28K. Arch Ital Biol. 1990;128:127–149. - PubMed
- Ben-Arie N, Bellen HJ, Armstrong DL, McCall AE, Gordadze PR, Guo Q, Matzuk MM, Zoghbi HY. Math1 is essential for genesis of cerebellar granule neurons. Nature. 1997;390:169–172. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous