Noninvasive volumetric imaging and morphometry of the rodent retina with high-speed, ultrahigh-resolution optical coherence tomography - PubMed (original) (raw)
Noninvasive volumetric imaging and morphometry of the rodent retina with high-speed, ultrahigh-resolution optical coherence tomography
Vivek J Srinivasan et al. Invest Ophthalmol Vis Sci. 2006 Dec.
Abstract
Purpose: To demonstrate high-speed, ultrahigh-resolution optical coherence tomography (OCT) for noninvasive, in vivo, three-dimensional imaging of the retina in rat and mouse models.
Methods: A high-speed, ultrahigh-resolution OCT system using spectral, or Fourier domain, detection has been developed for small animal retinal imaging. Imaging is performed with a contact lens and postobjective scanning. An axial image resolution of 2.8 mum is achieved with a spectrally broadband superluminescent diode light source with a bandwidth of approximately 150 nm at approximately 900-nm center wavelength. Imaging can be performed at 24,000 axial scans per second, which is approximately 100 times faster than previous ultrahigh-resolution OCT systems. High-definition and three-dimensional retinal imaging is performed in vivo in mouse and rat models.
Results: High-speed, ultrahigh-resolution OCT enabled high-definition, high transverse pixel density imaging of the murine retina and visualization of all major intraretinal layers. Raster scan protocols enabled three-dimensional volumetric imagingand comprehensive retinal segmentation algorithms allowed measurement of retinal layers. An OCT fundus image, akin to a fundus photograph was generated by axial summation of three-dimensional OCT data, thus enabling precise registration of OCT measurements to retinal fundus features.
Conclusions: High-speed, ultrahigh-resolution OCT enables imaging of retinal architectural morphology in small animal models. OCT fundus images allow precise registration of OCT images and repeated measurements with respect to retinal fundus features. Three-dimensional OCT imaging enables visualization and quantification of retinal structure, which promises to allow repeated, noninvasive measurements to track disease progression, thereby reducing the need for killing the animal for histology. This capability can accelerate basic research studies in rats and mice and their translation into clinical patient care.
Figures
Figure 1
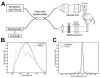
(A) Schematic of high-speed, ultrahigh-resolution OCT system for rat and mouse retinal imaging. Imaging is performed using contact lens and postobjective scanning. Spectral and Fourier domain detection are performed with a high-speed, high-resolution spectrometer. (B) Spectrum of the light source detected by the spectrometer, with and without numerical spectral shaping to smooth the spectrum. The compact, multiplexed superluminescent diode light source has BW = 145 nm at λc = 890 nm. (C) Axial PSF and without spectral shaping to reduce side lobes. The axial resolution is 2.8 _μ_m in tissue.
Figure 2
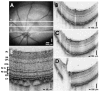
High-speed, ultrahigh-resolution OCT images from a normal C57BL6 mouse. (A) An OCT fundus image, created by axial summation of 3D-OCT data consisting of 256 images of 512 axial scans each, is shown. High-definition OCT images (B–D) with 2048 axial scans may be registered to the OCT fundus image. (E) Clear visualization of major intraretinal layers is enabled by OCT, as shown in the cropped, enlarged OCT image with ~600 axial scans.
Figure 3
High-speed, ultrahigh-resolution OCT images from a normal Long-Evans rat. (A) An OCT fundus image. High-definition images (B–D) with 2048 axial scans are registered to the fundus image. (E) Intraretinal layers are visualized in the cropped, enlarged OCT image with ~600 axial scans.
Figure 4
Using 3D-OCT data, visualization techniques such as volumetric rendering are possible. (A) A rendering of a normal Long-Evans rat retina. (B) It is possible to create virtual slices through 3D-OCT data and view images along arbitrary planes. Cut-away renderings can simultaneously show intraretinal structure and retinal topography (C).
Figure 5
Using 3D-OCT data, quantitative mapping of intraretinal layers is possible. (A) A fundus image of the Long-Evans rat retina. (B) Cross-sectional OCT images from the 3D-OCT data set are segmented to identify boundaries between retinal layers. Retinal (C) and NFL (D) thicknesses are shown in pseudocolor on the fundus image. The OCT fundus image (E) can be used for registration of baseline (blue) and repeated (red) measurements. This example shows repeated measurements performed 9 days apart. (F) Measurements of retinal thickness (using two different conventions), NFL, ONL, and photoreceptor OS thickness. Rows 1 to 2 and 4 to 5 (F) correspond to the white rectangular region (E), whereas row 3 (F) corresponds to the black rectangular region (E).
Figure 6
A comparison of cropped and enlarged OCT cross-sectional images (~600 axial scans) between normal, young-adult Sprague-Dawley (A) and Long-Evans (B) rats.
Figure 7
Comparisons between (A) representative histology and (B) OCT image in a young adult Long-Evans rat retina near the optic nerve head.
Similar articles
- Three-dimensional retinal imaging with high-speed ultrahigh-resolution optical coherence tomography.
Wojtkowski M, Srinivasan V, Fujimoto JG, Ko T, Schuman JS, Kowalczyk A, Duker JS. Wojtkowski M, et al. Ophthalmology. 2005 Oct;112(10):1734-46. doi: 10.1016/j.ophtha.2005.05.023. Ophthalmology. 2005. PMID: 16140383 Free PMC article. - High-definition and 3-dimensional imaging of macular pathologies with high-speed ultrahigh-resolution optical coherence tomography.
Srinivasan VJ, Wojtkowski M, Witkin AJ, Duker JS, Ko TH, Carvalho M, Schuman JS, Kowalczyk A, Fujimoto JG. Srinivasan VJ, et al. Ophthalmology. 2006 Nov;113(11):2054.e1-14. doi: 10.1016/j.ophtha.2006.05.046. Ophthalmology. 2006. PMID: 17074565 Free PMC article. - Ultrahigh-speed optical coherence tomography for three-dimensional and en face imaging of the retina and optic nerve head.
Srinivasan VJ, Adler DC, Chen Y, Gorczynska I, Huber R, Duker JS, Schuman JS, Fujimoto JG. Srinivasan VJ, et al. Invest Ophthalmol Vis Sci. 2008 Nov;49(11):5103-10. doi: 10.1167/iovs.08-2127. Epub 2008 Jul 24. Invest Ophthalmol Vis Sci. 2008. PMID: 18658089 Free PMC article. - State-of-the-art retinal optical coherence tomography.
Drexler W, Fujimoto JG. Drexler W, et al. Prog Retin Eye Res. 2008 Jan;27(1):45-88. doi: 10.1016/j.preteyeres.2007.07.005. Epub 2007 Aug 11. Prog Retin Eye Res. 2008. PMID: 18036865 Review. - The fundus photo has met its match: optical coherence tomography and adaptive optics ophthalmoscopy are here to stay.
Morgan JI. Morgan JI. Ophthalmic Physiol Opt. 2016 May;36(3):218-39. doi: 10.1111/opo.12289. Ophthalmic Physiol Opt. 2016. PMID: 27112222 Free PMC article. Review.
Cited by
- Adaptive optics retinal imaging in the living mouse eye.
Geng Y, Dubra A, Yin L, Merigan WH, Sharma R, Libby RT, Williams DR. Geng Y, et al. Biomed Opt Express. 2012 Apr 1;3(4):715-34. doi: 10.1364/BOE.3.000715. Epub 2012 Mar 15. Biomed Opt Express. 2012. PMID: 22574260 Free PMC article. - Developmental and morphological studies in Japanese medaka with ultra-high resolution optical coherence tomography.
Gladys FM, Matsuda M, Lim Y, Jackin BJ, Imai T, Otani Y, Yatagai T, Cense B. Gladys FM, et al. Biomed Opt Express. 2015 Jan 6;6(2):297-308. doi: 10.1364/BOE.6.000297. eCollection 2015 Feb 1. Biomed Opt Express. 2015. PMID: 25780725 Free PMC article. - Developing SDOCT to assess donor human eyes prior to tissue sectioning for research.
Brown NH, Koreishi AF, McCall M, Izatt JA, Rickman CB, Toth CA. Brown NH, et al. Graefes Arch Clin Exp Ophthalmol. 2009 Aug;247(8):1069-80. doi: 10.1007/s00417-009-1044-3. Epub 2009 Feb 19. Graefes Arch Clin Exp Ophthalmol. 2009. PMID: 19225801 Free PMC article. - Quantitative Analysis of Mouse Retinal Layers Using Automated Segmentation of Spectral Domain Optical Coherence Tomography Images.
Dysli C, Enzmann V, Sznitman R, Zinkernagel MS. Dysli C, et al. Transl Vis Sci Technol. 2015 Aug 25;4(4):9. doi: 10.1167/tvst.4.4.9. eCollection 2015 Aug. Transl Vis Sci Technol. 2015. PMID: 26336634 Free PMC article. - Activated Retinal Pigment Epithelium, an Optical Coherence Tomography Biomarker for Progression in Age-Related Macular Degeneration.
Curcio CA, Zanzottera EC, Ach T, Balaratnasingam C, Freund KB. Curcio CA, et al. Invest Ophthalmol Vis Sci. 2017 May 1;58(6):BIO211-BIO226. doi: 10.1167/iovs.17-21872. Invest Ophthalmol Vis Sci. 2017. PMID: 28785769 Free PMC article.
References
- Beaurepaire E, Boccara AC, Lebec M, Blanchot L, Saint-James H. Full-field optical coherence microscopy. Opt Lett. 1998;23:244–246. - PubMed
- Dubois A, Moneron G, Grieve K, Boccara AC. Three-dimensional cellular-level imaging using full-field optical coherence tomography. Phys Med Biol. 2004;49:1227–1234. - PubMed
- Hee MR, Izatt JA, Swanson EA, et al. Optical coherence tomography of the human retina. Arch Ophthalmol. 1995;113:325–332. - PubMed
- Puliafito CA, Hee MR, Lin CP, et al. Imaging of macular diseases with optical coherence tomography. Ophthalmology. 1995;102:217–229. - PubMed
Publication types
MeSH terms
Grants and funding
- R01-EY13178-06/EY/NEI NIH HHS/United States
- R01-EY11289-20/EY/NEI NIH HHS/United States
- R01 EY013178/EY/NEI NIH HHS/United States
- R01-EY12008-04/EY/NEI NIH HHS/United States
- R01 EY011289/EY/NEI NIH HHS/United States
- R01 EY012008/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources