Structural basis for ribosome recruitment and manipulation by a viral IRES RNA - PubMed (original) (raw)
Structural basis for ribosome recruitment and manipulation by a viral IRES RNA
Jennifer S Pfingsten et al. Science. 2006.
Abstract
Canonical cap-dependent translation initiation requires a large number of protein factors that act in a stepwise assembly process. In contrast, internal ribosomal entry sites (IRESs) are cis-acting RNAs that in some cases completely supplant these factors by recruiting and activating the ribosome using a single structured RNA. Here we present the crystal structures of the ribosome-binding domain from a Dicistroviridae intergenic region IRES at 3.1 angstrom resolution, providing a view of the prefolded architecture of an all-RNA translation initiation apparatus. Docking of the structure into cryo-electron microscopy reconstructions of an IRES-ribosome complex suggests a model for ribosome manipulation by a dynamic IRES RNA.
Figures
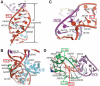
Fig. 1
IRES-driven initiation and the structure of the PSIV IGR IRES. (A) Ribosome recruitment strategies used in cap-dependent and IGR-IRES-dependent translation initiation. ORF, open reading frame; GTP, guanosine triphosphate; GDP, guanosine diphosphate. At right is the protein-independent pathway used by this IRES RNA. The inset is a cartoon of the secondary structure of the ribosome-binding domain, colored to match the structure of (B). Parts colored gray did not appear in the crystal structure and were not built into the final model. The secondary structure consists of two regions (regions 1 and 2), which contain two functionally critical pseudoknots (10, 12, 13). Because PK III is nested inside PK II, this forms an RNA tertiary structure called a double-nested pseudoknot (15). Figure S1 contains a detailed secondary structure with sequence information. (B) Structure of the ribosome-binding domain, colored to match the inset in (A). J, junction; P, paired/helix; L, loop. The gray hexagon shows structure that was weakly visible and hence conformationally flexible. The RNA crystallized in a domain-swapped dimer (fig. S4) in which the functionally essential structural features are preserved.
Fig. 2
Structural details of the IRES. (A) Underwound helix P2.2 (red) is shown from the minor groove side. A6105 (red) stacks into the helix, forming a noncanonical A·A N7-amino base pair with A6098 of J2.3 (cyan). A6135 (red) stacks on A6098 and forms a reverse (parallel) Watson-Crick A-U pair with conserved base U6097 of J2.3 (cyan). In this and subsequent panels, base pairing is indicated with double-ended arrows and stacking with a thick dashed line. (B) Within the P2.2 major groove, both U6097 (cyan) and U6082 (purple) pair with bases in P2.2 (red), whereas U6083 and U6096 pair with each other at the end of SL IV (cyan). Region 1 bases (green) buttress this structure through A-minor interactions. (C) The U6082-G6110 pairing extrudes U6130 from the helix and induces a sharp turn in the backbone. Bases A6129 and A6128 stack on the minor groove of the PK III helix, starting the base stacking that extends into SL V. (D) The two strands of L1.2 splay apart, with L1.2A lying in the minor groove of P2.2, stabilizing the inter-region packing through A-minor interactions (e.g., A6033 and A6034). Bases U6044 and U6045 of L1.2B continue to stack on the PK II stack, whereas A6046-A6048 form a u-turn-like structure (27), allowing A6049 and A6050 to reach the minor groove of P2.2.
Fig. 3
Interaction of the IGR IRES RNA with the ribosome. (A) The PSIV IGR IRES ribosome affinity domain structure docked into the cryo-EM representation of the IRES bound to the 80_S_ ribosome, with the 60_S_ ribosome density computationally removed. The 40_S_ subunit is in yellow, the cryo-EM density of the IRES is in gray, and the IRES crystal structure is colored as in Fig. 1. The positions of rpS5 (40_S_ subunit) and SL IV and SL V (IGR IRES) are shown. (B) Detailed view of the interaction of the IGR IRES to the 60_S_ subunit within the 80_S_ ribosome-IRES complex, with the 40_S_ subunit density computationally removed. The L1 stalk of the 60_S_ subunit contacts IRES loop L1.1 and perhaps P1.1. For both (A) and (B), the orientation is indicated in the insets.
Fig. 4
IRES structural changes and a proposed mechanism of ribosome recruitment. (A) Sequences of two L1.1/P1.1 mutants. COMP, Watson-Crick complement. (B) Native gel analysis of these mutants shows that they do not globally misfold, because they run very close to wild-type (WT) RNA. Previously published (10) native gel analysis of mutants, in which SL IV and V were changed to GAAA tetraloops (which does not change the fold) and in which PK II was altered (which causes global misfolding), is shown for comparison at right. (C) Assembly assays of L1.1/P1.1 mutants analyzed on a sucrose gradient. The locations of 40_S_- and 80_S_-bound IRESs are indicated. CPM, counts per minute. (D) Interactions with the 40_S_ subunit are shown in yellow, and those with the 60_S_ subunit are shown in blue. The IRES is shown as a space-filling representation inside the corresponding gray cryo-EM density, colored as in previous figures. The folded, unbound IRES binds to the 40_S_ subunit through SL IV and SL V interacting with rpS5, inducing a conformational change in the subunit and docking region 3 into the P site (step 1). Subsequent 60_S_ subunit binding induces a series of conformational changes in both the IRES and the ribosome, including a putative organization of IRES structures L1.1 and P1.1 (step 2). The appearance of additional IRES density in the cryo-EM map of the 80_S_ ribosome-bound IRES (indicated with the red oval) supports the existence of this structural switch.
Similar articles
- Structure of the ribosome-bound cricket paralysis virus IRES RNA.
Schüler M, Connell SR, Lescoute A, Giesebrecht J, Dabrowski M, Schroeer B, Mielke T, Penczek PA, Westhof E, Spahn CM. Schüler M, et al. Nat Struct Mol Biol. 2006 Dec;13(12):1092-6. doi: 10.1038/nsmb1177. Epub 2006 Nov 19. Nat Struct Mol Biol. 2006. PMID: 17115051 - Factorless ribosome assembly on the internal ribosome entry site of cricket paralysis virus.
Jan E, Sarnow P. Jan E, et al. J Mol Biol. 2002 Dec 13;324(5):889-902. doi: 10.1016/s0022-2836(02)01099-9. J Mol Biol. 2002. PMID: 12470947 - Cryo-EM visualization of a viral internal ribosome entry site bound to human ribosomes: the IRES functions as an RNA-based translation factor.
Spahn CM, Jan E, Mulder A, Grassucci RA, Sarnow P, Frank J. Spahn CM, et al. Cell. 2004 Aug 20;118(4):465-75. doi: 10.1016/j.cell.2004.08.001. Cell. 2004. PMID: 15315759 - Dynamics of IRES-mediated translation.
Johnson AG, Grosely R, Petrov AN, Puglisi JD. Johnson AG, et al. Philos Trans R Soc Lond B Biol Sci. 2017 Mar 19;372(1716):20160177. doi: 10.1098/rstb.2016.0177. Philos Trans R Soc Lond B Biol Sci. 2017. PMID: 28138065 Free PMC article. Review. - Comparing the three-dimensional structures of Dicistroviridae IGR IRES RNAs with other viral RNA structures.
Kieft JS. Kieft JS. Virus Res. 2009 Feb;139(2):148-56. doi: 10.1016/j.virusres.2008.07.007. Epub 2008 Aug 15. Virus Res. 2009. PMID: 18672012 Free PMC article. Review.
Cited by
- An innovative protein expression system using RNA polymerase I for large-scale screening of high-nucleic-acid content Saccharomyces cerevisiae strains.
Zeng D, Qiu C, Shen Y, Hou J, Li Z, Zhang J, Liu S, Shang J, Qin W, Xu L, Bao X. Zeng D, et al. Microb Biotechnol. 2020 Nov;13(6):2008-2019. doi: 10.1111/1751-7915.13653. Epub 2020 Aug 27. Microb Biotechnol. 2020. PMID: 32854170 Free PMC article. - Predicting pseudoknotted structures across two RNA sequences.
Sperschneider J, Datta A, Wise MJ. Sperschneider J, et al. Bioinformatics. 2012 Dec 1;28(23):3058-65. doi: 10.1093/bioinformatics/bts575. Epub 2012 Oct 8. Bioinformatics. 2012. PMID: 23044552 Free PMC article. - Non-Canonical Translation Initiation Mechanisms Employed by Eukaryotic Viral mRNAs.
Sorokin II, Vassilenko KS, Terenin IM, Kalinina NO, Agol VI, Dmitriev SE. Sorokin II, et al. Biochemistry (Mosc). 2021 Sep;86(9):1060-1094. doi: 10.1134/S0006297921090042. Biochemistry (Mosc). 2021. PMID: 34565312 Free PMC article. Review. - Host-like RNA Elements Regulate Virus Translation.
Khan D, Fox PL. Khan D, et al. Viruses. 2024 Mar 20;16(3):468. doi: 10.3390/v16030468. Viruses. 2024. PMID: 38543832 Free PMC article. Review. - Molecular biology: signals across domains of life.
Jan E. Jan E. Nature. 2015 Mar 5;519(7541):40-1. doi: 10.1038/nature14202. Epub 2015 Feb 4. Nature. 2015. PMID: 25652822 No abstract available.
References
- Hershey JWB, Merrick WC. In: Translational Control of Gene Expression. Sonenberg N, Hershey JWB, Mathews MB, editors. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2000. pp. 33–88.
- Hellen CU, Sarnow P. Genes Dev. 2001;15:1593. - PubMed
- Jan E. Virus Res. 2005;119:16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM072560/GM/NIGMS NIH HHS/United States
- R01 GM072560-03/GM/NIGMS NIH HHS/United States
- R01 GM072560-01/GM/NIGMS NIH HHS/United States
- R01 GM072560-04/GM/NIGMS NIH HHS/United States
- R01 GM072560-05/GM/NIGMS NIH HHS/United States
- R01 GM072560-02/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous