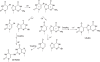Sequence-dependent formation of intrastrand crosslink products from the UVB irradiation of duplex DNA containing a 5-bromo-2'-deoxyuridine or 5-bromo-2'-deoxycytidine - PubMed (original) (raw)
Sequence-dependent formation of intrastrand crosslink products from the UVB irradiation of duplex DNA containing a 5-bromo-2'-deoxyuridine or 5-bromo-2'-deoxycytidine
Yu Zeng et al. Nucleic Acids Res. 2006.
Abstract
The replacement of thymidine with 5-bromo-2'-deoxyuridine (BrdU) is well-known to sensitize cells to ionizing radiation and photoirradiation. We reported here the sequence-dependent formation of intrastrand crosslink products from the UVB irradiation of duplex oligodeoxynucleotides harboring a BrdU or its closely related 5-bromo-2'-deoxycytidine (BrdC). Our results showed that two types of crosslink products could be induced from d(BrCG), d(BrUG), d(GBrU), or d(ABrU); the C(5) of cytosine or uracil could be covalently bonded to the N(2) or C(8) of its neighboring guanine, and the C(5) of uracil could couple with the C(2) or C(8) of its neighboring adenine. By using those crosslink product-bearing dinucleoside monophosphates as standards, we demonstrated, by using liquid chromatography-mass spectrometry/mass spectrometry (LC-MS/MS), that all the crosslink products described above except d(G[N(2)-5]U) and d(G[N(2)-5]C) could form in duplex DNA. In addition, LC-MS/MS quantification results revealed that both the nature of the halogenated pyrimidine base and its 5' flanking nucleoside affected markedly the generation of intrastrand crosslink products. The yields of crosslink products were much higher while the 5' neighboring nucleoside was a dG than while it was a dA, and BrdC induced the formation of crosslink products much more efficiently than BrdU. The formation of intrastrand crosslink products from these halopyrimidines in duplex DNA may account for the photosensitizing effects of these nucleosides.
Figures
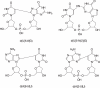
Scheme 1
Intrastrand crosslink products formed from d(BrUG) and d(ABrU).
Scheme 2
Proposed mechanisms for the formation of intrastrand crosslink products between uracil and guanine.
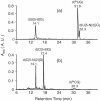
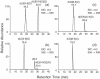
Figure 1
HPLC traces for the separation of the aerobic irradiation mixtures of d(BrUG) (a) and d(BrCG) (b).
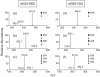
Figure 2
Positive-ion product-ion spectra of d(U[5-8]G): MS/MS (a), MS3 (b), MS4 (c) and d(G[8-5]U): MS/MS (d), MS3 (e), MS4 (f). The relative collisional energies were 30%.
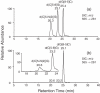
Figure 3
LC-MS/MS analysis of the standard d(U ^ G) (a) and d(G ^ U) (c) crosslink products and the enzymatic digestion products of the aerobic UVB-irradiated duplex ODN d(ATGGCGBrUGCTAT)/d(ATAGCACGCCAT) (b and d). Shown are the SICs for the monitoring of the m/z 556→538 (a and b) and m/z 556→458 (c and d) transitions.
Figure 4
Positive-ion product-ion spectra of d(C[5-N(2)]G): MS/MS (a), MS3 (d); d(C[5-8]G): MS/MS (b), MS3 (e); and d(G[8-5]C): MS/MS (c), MS3(f). The relative collisional energies were 30%.
Figure 5
LC-MS/MS analysis of the standard d(C ^ G) and d(G ^ C) crosslink products (a) and the enzymatic digestion products of the UVB-irradiated duplex ODN d(ATGGCGBrCGCTAT)/d(ATAGCGCGCCAT) (b). Shown are the SICs for monitoring the m/z 555→261 transition. A portion of SIC for the analysis of the digestion products was plotted in a different scale to better view the formation of the two C ^ G crosslinks [inset in (b)].
Similar articles
- UVB-Induced formation of intrastrand cross-link products of DNA in MCF-7 cells treated with 5-bromo-2'-deoxyuridine.
Zeng Y, Wang Y. Zeng Y, et al. Biochemistry. 2007 Jul 10;46(27):8189-95. doi: 10.1021/bi700431q. Epub 2007 Jun 13. Biochemistry. 2007. PMID: 17567044 - 5-Bromo-2'-deoxycytidine-a potential DNA photosensitizer.
Zdrowowicz M, Wityk P, Michalska B, Rak J. Zdrowowicz M, et al. Org Biomol Chem. 2016 Oct 4;14(39):9312-9321. doi: 10.1039/c6ob01446a. Org Biomol Chem. 2016. PMID: 27714178 - DHPLC and MS studies of a photoinduced intrastrand cross-link in DNA labeled with 5-bromo-2'-deoxyuridine.
Wiczk J, Miloch J, Rak J. Wiczk J, et al. J Photochem Photobiol B. 2014 Jan 5;130:86-92. doi: 10.1016/j.jphotobiol.2013.11.004. Epub 2013 Nov 14. J Photochem Photobiol B. 2014. PMID: 24300995 - Fragmentation of isomeric intrastrand crosslink lesions of DNA in an ion-trap mass spectrometer.
Cao H, Wang Y. Cao H, et al. J Am Soc Mass Spectrom. 2009 Apr;20(4):611-7. doi: 10.1016/j.jasms.2008.11.020. Epub 2008 Dec 6. J Am Soc Mass Spectrom. 2009. PMID: 19103496 Free PMC article.
Cited by
- Occurrence, Biological Consequences, and Human Health Relevance of Oxidative Stress-Induced DNA Damage.
Yu Y, Cui Y, Niedernhofer LJ, Wang Y. Yu Y, et al. Chem Res Toxicol. 2016 Dec 19;29(12):2008-2039. doi: 10.1021/acs.chemrestox.6b00265. Epub 2016 Nov 7. Chem Res Toxicol. 2016. PMID: 27989142 Free PMC article. Review. - Nuclease digestion and mass spectrometric characterization of oligodeoxyribonucleotides containing 1,2-GpG, 1,2-ApG, and 1,3-GpXpG cisplatin intrastrand cross-links.
Williams RT, Nalbandian JN, Tu A, Wang Y. Williams RT, et al. Clin Chim Acta. 2013 May;420:160-70. doi: 10.1016/j.cca.2012.12.010. Epub 2012 Dec 22. Clin Chim Acta. 2013. PMID: 23266768 Free PMC article. - Conformation-dependent formation of the G[8-5]U intrastrand cross-link in 5-bromouracil-containing G-quadruplex DNA induced by UVA irradiation.
Lin G, Zhang J, Zeng Y, Luo H, Wang Y. Lin G, et al. Biochemistry. 2010 Mar 23;49(11):2346-50. doi: 10.1021/bi901861w. Biochemistry. 2010. PMID: 20166754 Free PMC article. - Chemical and structural characterization of interstrand cross-links formed between abasic sites and adenine residues in duplex DNA.
Price NE, Catalano MJ, Liu S, Wang Y, Gates KS. Price NE, et al. Nucleic Acids Res. 2015 Apr 20;43(7):3434-41. doi: 10.1093/nar/gkv174. Epub 2015 Mar 16. Nucleic Acids Res. 2015. PMID: 25779045 Free PMC article. - UVA photoactivation of DNA containing halogenated thiopyrimidines induces cytotoxic DNA lesions.
Brem R, Zhang X, Xu YZ, Karran P. Brem R, et al. J Photochem Photobiol B. 2015 Apr;145:1-10. doi: 10.1016/j.jphotobiol.2015.02.012. Epub 2015 Feb 20. J Photochem Photobiol B. 2015. PMID: 25747491 Free PMC article.
References
- Dewey W.C., Humphrey R.M. Increase in radiosensitivity to ionizing radiation related to replacement of thymidine in mammalian cells with 5-bromodeoxyuridine. Radiat. Res. 1965;26:538–553. - PubMed
- Sano K., Hoshino T., Nagai M. Radiosensitization of brain tumor cells with a thymidine analogue (bromouridine) J. Neurosurg. 1968;28:530–538. - PubMed
- Ling L.L., Ward J.F. Radiosensitization of Chinese hamster V79 cells by bromodeoxyuridine substitution of thymidine: enhancement of radiation-induced toxicity and DNA strand break production by monofilar and bifilar substitution. Radiat. Res. 1990;121:76–83. - PubMed
- Webb C.F., Jone G.D., Moyer D.J., Aguilera J.A., Ling L.L. Mechanisms of radiosensitization in bromodeoxyuridine-substituted cells. Int. J. Radiat. Biol. 1993;64:695–705. - PubMed
- Taguchi T., Shiraishi Y. Increased sister-chromatid exchanges (SCEs) and chromosomal fragilities by BrdU in a human mutant B-lymphoblastoid cell line. Mutat. Res. 1989;211:43–49. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous