Wnt11 controls cell contact persistence by local accumulation of Frizzled 7 at the plasma membrane - PubMed (original) (raw)
Wnt11 controls cell contact persistence by local accumulation of Frizzled 7 at the plasma membrane
Sabine Witzel et al. J Cell Biol. 2006.
Abstract
Wnt11 is a key signal, determining cell polarization and migration during vertebrate gastrulation. It is known that Wnt11 functionally interacts with several signaling components, the homologues of which control planar cell polarity in Drosophila melanogaster. Although in D. melanogaster these components are thought to polarize cells by asymmetrically localizing at the plasma membrane, it is not yet clear whether their subcellular localization plays a similarly important role in vertebrates. We show that in zebrafish embryonic cells, Wnt11 locally functions at the plasma membrane by accumulating its receptor, Frizzled 7, on adjacent sites of cell contacts. Wnt11-induced Frizzled 7 accumulations recruit the intracellular Wnt signaling mediator Dishevelled, as well as Wnt11 itself, and locally increase cell contact persistence. This increase in cell contact persistence is mediated by the local interaction of Wnt11, Frizzled 7, and the atypical cadherin Flamingo at the plasma membrane, and it does not require the activity of further downstream effectors of Wnt11 signaling, such as RhoA and Rok2. We propose that Wnt11, by interacting with Frizzled 7 and Flamingo, modulates local cell contact persistence to coordinate cell movements during gastrulation.
Figures
Figure 1.
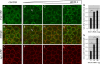
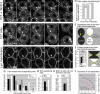
Wnt11 induces local accumulation of Fz7 at the plasma membrane. (A–L) Coexpression of fz7-yfp in green and membrane marker lyn-cfp in red (60 pg mRNA each) in the animal pole assay (pregastrula-stage embryos, 5 hpf) in the absence (A–C) and presence (D–L) of 5 (D–F), 10 (G–I), or 20 pg (J–L) of wnt11 mRNA. Arrowheads mark Fz7 accumulation at the plasma membrane. (M) Ratio of Fz7-YFP accumulations at the plasma membrane/cell contact number (2D quantification; 70 cells total from seven embryos were used per condition). (N) Ratio of Fz7-YFP accumulation length/total cell contact length. Note that in control cells, Fz7-YFP accumulations were never seen (70 measurements total from six embryos were used per condition). Error bars represent the SEM. Asterisks demarcate statistically significant differences (P < 0.05). Bar, 10 μm.
Figure 2.
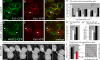
Wnt11 locally accumulates with Fz7 at the plasma membrane. (A–F) Coexpression of wnt11-yfp (50–100 pg mRNA) with lyn-cfp (60 pg mRNA; red; A–C) or fz7-cfp (110 pg mRNA; red; D–F) in the animal pole assay. Arrowheads mark Wnt11-YFP puncta at the plasma membrane (A–C) and Wnt11-YFP/Fz7-CFP–positive accumulations (D–F). (G–L) Wnt11-YFP–expressing (green) cells transplanted into host embryos expressing either Lyn-CFP (red; G–I) or Fz7-YFP (red; J–L). Arrowheads mark Wnt11-YFP puncta at host cell membrane (G–I) or Fz7-CFP/Wnt11-YFP–positive accumulations at host cell membrane. Bars: 10 μm.
Figure 3.
Wnt11-induced Fz7 accumulations form on contacting plasma membranes. (A–F) As diagramed (A and B), cells expressing fz7-yfp (60 pg mRNA; red) were transplanted into the animal pole of host embryos (4 hpf) coexpressing fz7-cfp (110 pg mRNA; green) and wnt11 (20 pg mRNA) and imaged 1–3 h later. (C) Fz7-YFP accumulation (arrowhead) between transplanted donor cells induced by Wnt11 expressed in surrounding host cells. (D–F) Fz7 accumulation between donor and host cells form at both donor cell (red) and host cell membranes (arrowheads). Bars, 10 μm.
Figure 4.
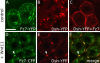
Dsh is recruited to Wnt11-induced Fz7 accumulations. (A–F) Animal pole assay in fixed embryos expressing either _fz7_-yfp (60 pg mRNA; A), dsh-yfp (75 pg mRNA; B), a combination of dsh-yfp and fz7 (50 pg mRNA; C), or a combination of dsh-yfp, _fz7_-cfp (110 pg mRNA) and wnt11 (20 pg mRNA; D–F). Arrowheads mark Fz7-CFP accumulations colocalizing with Dsh-YFP. Bar, 10 μm.
Figure 5.
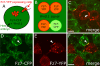
Wnt11-induced accumulations of Fz7 modulate cell contact persistence. (A–C) Time-series of separating cells in the animal pole assay expressing either a combination of fz7-yfp (60 pg mRNA) and wnt11 (10 pg mRNA; A; Video 1), fz7-yfp alone (60 pg mRNA; B; Video 3), or lyn-yfp (25 pg mRNA; C; Video 4). Cells were “back-tracked” from the time point of separation (0′). Z sections shown from 3D, two-photon imaging. Time intervals are 130 (A and B) and 123 s (C); contact sites are marked by arrowheads. (D) Ratio of length of Fz7 accumulation/total cell contact length at the first video time point and last time point before separation (n = 26 out of 4 videos). Error bars represent the SEM. (E) Diagram of contacting cells containing Lyn-YFP or Fz7-YFP (control, black) versus Wnt11 + Fz7-YFP (white) at the last time point before separation. (F) Average contact angle between contacting cells at the last time point before separation. Number of cells analyzed was as follows: 34 (Fz7), 31 (Lyn), and 27 (Fz7 + Wnt11) out of 4 videos per condition. Asterisks mark statistically significant differences (P < 0.05). (G) Relative distribution of contact times from randomly chosen pairs of separating cells measured from start of the time-lapse until cell separation. Number of cells analyzed was as follows: 38 (Fz7), 32 (Lyn), and 25 (Fz7 + Wnt11) out of 4 videos per condition. (G′) Percentage of cells not separating within a 75-min time frame. Number of cells analyzed was as follows: 42 (Fz7), 43 (Lyn), and 36 (Fz7 + Wnt11) out of 4 videos each. (H) Ratio of cells contacting for >30 min (including nonseparating cells)/cells separating for <30 min from start of video. Calculated from data shown in G and G′. (I) Dynamic reduction of cell contact length during separation (Lyn, black; Fz7, gray; Fz7 + Wnt11, white) and reduction of Fz7 accumulation length (Fz7 + Wnt11, red). Graphs represent an average of all separation events measured per condition. Number of cells analyzed was as follows: 19 (Fz7), 26 (Lyn), and 23 (Fz7 + Wnt11) out of 4 videos per condition. t = 0 min is the first time point of separation. Bars, 10 μm. Videos 1, 3, and 4 are available at
http://www.jcb.org/cgi/content/full/jcb.200606017/DC1
.
Figure 6.
Fmi2 colocalizes with Fz7 and Wnt11 at cell contacts and increases persistence. (A–F) Animal pole assay in fixed embryos expressing a mix of fz7-cfp (110 pg mRNA) and fmi2-yfp (50 pg DNA; A–C), or a mix of wnt11-cfp (90 pg mRNA), fz7 (50 pg mRNA), and fmi2-yfp (50 pg DNA; D–F). (A–C) Fz7-CFP (green) and Fmi2-YFP (red) colocalize in accumulations (arrowheads) at cell contacts. (D–F) Wnt11-CFP (green) and Fmi2-YFP (red) in the presence of exogenous Fz7 colocalize in accumulations (arrowheads) at cell contacts. (G) Time series of separating cells expressing Fmi2-YFP in the animal pole assay (Video 5). Fmi2-YFP accumulates (arrowheads) and remains at contacts until cell separation. Time series was obtained as described in Fig. 5, at time intervals of 171 s. (H) Relative distribution of cell contact times for separating cells expressing either fmi2-yfp (black), wnt11 + fz7-yfp (white), a combination of wnt11 + fz7-yfp, and MOs targeted against fmi1a, fmi1b, and fmi2 (4 ng/MO; gray; Video 6). Cell contact times were measured as described in Fig. 5. Number of cells analyzed was as follows: 24 (Fmi2), 25 (Fz7 + Wnt11), and 29 (Fz7 + Wnt11 + FmiMO) out of 4 videos per condition. (H′) Percentage of cells not separating within 75 min. Number of cells analyzed was as follows: 40 (Fmi2), 36 (Fz7 + Wnt11), and 35 (Fz7 + Wnt11 + FmiMO) out of 4 videos per condition. (H") Ratio of cells contacting for >30 min (including nonseparating cells)/cells separating for <30 min from start of video. Calculated from data shown in H and H′. (I and I′) Dynamic reduction of cell contact length during separation (I′), measured as described in Fig. 5. Number of cells analyzed was as follows: 19 (Fz7), 26 (Lyn), 23 (Fz7 + Wnt11), 27 (Fz7 + Wnt11 + FmiMO), and 25 (Fmi2) out of 4 videos per condition. Velocity of cell contact shrinkage (I) representing the slope of the last time point before separation to t = 0 in I′. Asterisks mark significant difference with P < 0.05. Error bars represent the SEM. Bars, 10 μm. Videos 5 and 6 are available at
http://www.jcb.org/cgi/content/full/jcb.200606017/DC1
.
Figure 7.
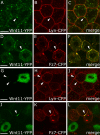
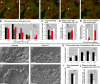
Endogenous subcellular sites of Wnt11 and Fz7 activity marked by Dsh-YFP recruitment to the plasma membrane. (A–C) Epiblast cells in germ ring of fixed gastrulating embryos (shield stage, 6 hpf) expressing dsh-yfp (75 pg mRNA) and lyn-cfp (75 pg mRNA). Dsh-YFP (green) localized in puncta (arrowheads) at the plasma membrane (red) in wild-type (A), slb/wnt11 mutant (B), and “rescued” slb/wnt11 mutant embryos (C; coinjected with 20 pg wnt11 mRNA). (D) Number of Dsh-YFP puncta at the plasma membrane (for rescue condition, column represents both epiblast and hypoblast cells). Quantification performed in 3D by counting the number of Dsh-YFP puncta at the plasma membrane of a chosen cell in all z sections (step size = 1.5 μm). Number of cells analyzed was as follows (epiblast/hypoblast): 50/43 (wild type); 43/43 (slb/wnt11 mutants); 83 (slb/wnt11 rescue; epiblast + hypoblast) out of 6 embryos per condition. Error bars represent the SEM. (E–G′) Epiblast cells in the germ ring of living embryos (6 hpf) expressing dsh-cfp (100 pg mRNA), and mosaic wnt11-yfp mRNA (50–100 pg mRNA injection at the 16-cell–stage). Plasma membranes stained (E′–G′; blue) with FM464 by intercellular injection at 5 hpf. Wnt11-YFP (E, E′, G, and G′; green) and Dsh-CFP (F, F′, G, and G′; red) colocalize in puncta (arrowheads) at the plasma membrane (blue) of both Wnt11-producing (green cytoplasm) and receiving cells. (H–L) As diagramed (H and I), cells expressing dsh-yfp (60 pg mRNA; red) were transplanted into the animal pole of host embryos (4 hpf) coexpressing dsh-cfp (100 pg mRNA; green) and wnt11 (50 pg mRNA). FM464 marks plasma membrane (blue). Epiblast cells imaged 3 h after transplantation within the germ ring of a living host embryo (6 hpf). (J–L) Dsh-YFP of a transplanted cell and Dsh-CFP in adjacent host cell colocalize to the same site (arrowheads). Bars, 10 μm.
Figure 8.
Wnt11 persist at cell contacts and is required for contact persistence. (A) Time series of separating hypoblast cells expressing a mix of 80 pg of wnt11-yfp and GPI-anchored rfp (mem-rfp) mRNA in shield-stage embryos (6 hpf). Wnt11-YFP (green) located in puncta at cell contacts (arrowheads) and remained until separation (Video 7). Time-series was obtained as in Fig. 5; time interval = 112 s. (B–E) Quantification of separation behavior of cells expressing Wnt11-YFP and mem-RFP (as in A, red) in animal pole assay (Video 8) compared with conditions described in Fig. 5 (G–H; Fz7-YFP + Wnt11, white; Lyn-YFP, black; Fz7-YFP, gray). (B) Relative distribution of contact times produced, as described for Fig. 5. Number of cells analyzed was as follows: 28 (Wnt11), 25 (Fz7 + Wnt11), 38 (Fz7), and 32 (Lyn) out of 4 videos per condition. (C) Percentage of cells not separating in 75 min. Number of cells analyzed was as follows: 36 (Wnt11), 36 (Fz7 + Wnt11), 42 (Fz7), and 43 (Lyn) out of 4 videos per condition. (D) Ratio of cells contacting for >30 min (including nonseparating cells)/cells separating for <30 min from start of video. Calculated from data in B and C. (E) Ratio of length of Wnt11 puncta or Fz7 accumulation/total contact length at the first video time point and the last time point before separation; number of cells analyzed was as follows: 20 (Wnt11) and 26 (Fz7 + Wnt11) out of 4 videos per condition. (F–G′) Images of 3D time-lapse videos recorded for 75-min and 30-s time-intervals in wild-type (F and F′, and Video 9) and _slb/wnt11_ (G and G′, and Video 10) mutant embryos at late shield stage (7 hpf), using bright-field microscopy. "Head-on" view of the dorsal germ ring (shield) showing cells at the leading edge of the prechordal plate. Images shown are first (F and G) and last (F′ and G′) video time points. Numbers mark exemplary cells that separate (white) or remain in contact (black). (H) Relative distribution of cell contact times for separating wild-type and _slb/wnt11_ mutant prechordal plate progenitors (see Videos 9 and 10). Measured in 3D and presented as described for Fig. 5. Number of cells analyzed was as follows: 33 (wild type) and 37 (_slb/wnt11_) out of 5 videos per condition. (I) Percentage of cells not separating in wild-type and _slb/wnt11_ mutant embryos in 75 min. Number of cells analyzed was as follows: 42 (wild type) and 44 (_slb/wnt11_) out of 5 videos per condition. (J) Ratio of cells contacting >30 min (including nonseparating cells)/cells separating <30 min from start of video. Calculated from data in H and I. Asterisk represents the significant difference, with P < 0.05. Error bars represent the SEM. Bars: (A) 10 μm; (F, F′, G, and G′) 50 μm. Videos 8–10 are available at
http://www.jcb.org/cgi/content/full/jcb.200606017/DC1
.
Similar articles
- Ror2 receptor mediates Wnt11 ligand signaling and affects convergence and extension movements in zebrafish.
Bai Y, Tan X, Zhang H, Liu C, Zhao B, Li Y, Lu L, Liu Y, Zhou J. Bai Y, et al. J Biol Chem. 2014 Jul 25;289(30):20664-76. doi: 10.1074/jbc.M114.586099. J Biol Chem. 2014. PMID: 24928507 Free PMC article. - Flamingo regulates epiboly and convergence/extension movements through cell cohesive and signalling functions during zebrafish gastrulation.
Carreira-Barbosa F, Kajita M, Morel V, Wada H, Okamoto H, Martinez Arias A, Fujita Y, Wilson SW, Tada M. Carreira-Barbosa F, et al. Development. 2009 Feb;136(3):383-92. doi: 10.1242/dev.026542. Epub 2008 Dec 17. Development. 2009. PMID: 19091770 Free PMC article. - The Fz-Dsh planar cell polarity pathway induces oriented cell division via Mud/NuMA in Drosophila and zebrafish.
Ségalen M, Johnston CA, Martin CA, Dumortier JG, Prehoda KE, David NB, Doe CQ, Bellaïche Y. Ségalen M, et al. Dev Cell. 2010 Nov 16;19(5):740-52. doi: 10.1016/j.devcel.2010.10.004. Dev Cell. 2010. PMID: 21074723 Free PMC article. - Dishevelled: The hub of Wnt signaling.
Gao C, Chen YG. Gao C, et al. Cell Signal. 2010 May;22(5):717-27. doi: 10.1016/j.cellsig.2009.11.021. Epub 2009 Dec 13. Cell Signal. 2010. PMID: 20006983 Review. - Frizzled signalling and cell polarisation in Drosophila and vertebrates.
Strutt D. Strutt D. Development. 2003 Oct;130(19):4501-13. doi: 10.1242/dev.00695. Development. 2003. PMID: 12925579 Review.
Cited by
- Defining the gene repertoire and spatiotemporal expression profiles of adhesion G protein-coupled receptors in zebrafish.
Harty BL, Krishnan A, Sanchez NE, Schiöth HB, Monk KR. Harty BL, et al. BMC Genomics. 2015 Feb 8;16(1):62. doi: 10.1186/s12864-015-1296-8. BMC Genomics. 2015. PMID: 25715737 Free PMC article. - Wnt/planar cell polarity signaling controls morphogenetic movements of gastrulation and neural tube closure.
Shi DL. Shi DL. Cell Mol Life Sci. 2022 Nov 12;79(12):586. doi: 10.1007/s00018-022-04620-8. Cell Mol Life Sci. 2022. PMID: 36369349 Free PMC article. Review. - Sfrp5 coordinates foregut specification and morphogenesis by antagonizing both canonical and noncanonical Wnt11 signaling.
Li Y, Rankin SA, Sinner D, Kenny AP, Krieg PA, Zorn AM. Li Y, et al. Genes Dev. 2008 Nov 1;22(21):3050-63. doi: 10.1101/gad.1687308. Genes Dev. 2008. PMID: 18981481 Free PMC article. - Molecular basis of morphogenesis during vertebrate gastrulation.
Wang Y, Steinbeisser H. Wang Y, et al. Cell Mol Life Sci. 2009 Jul;66(14):2263-73. doi: 10.1007/s00018-009-0018-2. Epub 2009 Apr 4. Cell Mol Life Sci. 2009. PMID: 19347571 Free PMC article. Review. - A role for planar cell polarity signaling in angiogenesis.
Cirone P, Lin S, Griesbach HL, Zhang Y, Slusarski DC, Crews CM. Cirone P, et al. Angiogenesis. 2008;11(4):347-60. doi: 10.1007/s10456-008-9116-2. Epub 2008 Sep 17. Angiogenesis. 2008. PMID: 18798004 Free PMC article.
References
- Adler, P.N. 2002. Planar signaling and morphogenesis in Drosophila. Dev. Cell. 2:525–535. - PubMed
- Classen, A.K., K.I. Anderson, E. Marois, and S. Eaton. 2005. Hexagonal packing of Drosophila wing epithelial cells by the planar cell polarity pathway. Dev. Cell. 9:805–817. - PubMed
- Djiane, A., J. Riou, M. Umbhauer, J. Boucaut, and D. Shi. 2000. Role of frizzled 7 in the regulation of convergent extension movements during gastrulation in Xenopus laevis. Development. 127:3091–3100. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases