Regulation of emotional responses elicited by threat-related stimuli - PubMed (original) (raw)
Regulation of emotional responses elicited by threat-related stimuli
Falk Eippert et al. Hum Brain Mapp. 2007 May.
Abstract
The capacity to voluntarily regulate emotions is critical for mental health, especially when coping with aversive events. Several neuroimaging studies of emotion regulation found the amygdala to be a target for downregulation and prefrontal regions to be associated with downregulation. To characterize the role of prefrontal regions in bidirectional emotion regulation and to investigate regulatory influences on amygdala activity and peripheral physiological measures, a functional magnetic resonance imaging (fMRI) study with simultaneous recording of self-report, startle eyeblink, and skin conductance responses was carried out. Subjects viewed threat-related pictures and were asked to up- and downregulate their emotional responses using reappraisal strategies. While startle eyeblink responses (in successful regulators) and skin conductance responses were amplified during upregulation, but showed no consistent effect during downregulation, amygdala activity was increased and decreased according to the regulation instructions. Trial-by-trial ratings of regulation success correlated positively with activity in amygdala during upregulation and orbitofrontal cortex during downregulation. Downregulation was characterized by left-hemispheric activation peaks in anterior cingulate cortex, dorsolateral prefrontal cortex, and orbitofrontal cortex and upregulation was characterized by a pattern of prefrontal activation not restricted to the left hemisphere. Further analyses showed significant overlap of prefrontal activation across both regulation conditions, possibly reflecting cognitive processes underlying both up- and downregulation, but also showed distinct activations in each condition. The present study demonstrates that amygdala responses to threat-related stimuli can be controlled through the use of cognitive strategies depending on recruitment of prefrontal areas, thereby changing the subject's affective state.
Figures
Figure 1
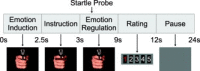
Experimental paradigm. Pictures were presented for 2.5 s, after which the regulation instruction (increase, decrease, or view) appeared in the center of the picture for 0.5 s. From this point on subjects were to regulate their emotions for 6 s; at 2 s into the regulation phase an acoustic startle probe was delivered. After the regulation phase subjects had to rate their success in regulation on a scale from 1–5 by button presses. Before the next trial began a gray square appeared, indicating the subjects to relax. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.\]
Figure 2
Subjects' threat and disgust ratings. Directly after scanning, subjects viewed all previously seen pictures again and indicated for each picture whether it was perceived as frightening/threatening, disgusting, or not eliciting an emotion; 64% of the negative pictures were rated as frightening/threatening by more than 50% of the subjects, while only 14% of the negative pictures were rated as disgusting by more than 50% of the subjects.
Figure 3
Startle eyeblink and SCR amplitudes in the regulation phase. Upregulation (increase) significantly enhanced startle eyeblink and SCR amplitudes in comparison to the condition of viewing the pictures. Downregulation (decrease) showed a nonsignificant attenuation of startle eyeblink responses and a trend toward a significant enhancement of SCR amplitudes. Startle amplitudes are depicted separately for increase‐view and decrease‐view because each graph depicts only successful regulators in that condition as determined by success ratings. Error bars denote standard error of the mean. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.\]
Figure 4
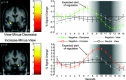
Amygdala activation in the view‐minus‐decrease (top) and increase‐minus‐view (bottom) contrasts and corresponding time‐courses. In comparison to simply viewing the pictures, left amygdala activity was significantly downregulated when decreasing and right amygdala activity was significantly upregulated when increasing (left amygdala activity was also upregulated, but this cannot be seen on this coronal section). Activations are overlaid on subjects' mean anatomy at a level of P < 0.001 uncorrected (for visualization, images were masked by the amygdala region of interest mask); color scales denote _t_‐values. To depict temporal characteristics of amygdala activation, time‐courses were extracted from a 6‐mm sphere around the highest activated voxel; error bars in time‐courses denote standard error of the mean. The gray background represents the time range in which effects of regulation were expected. Assuming that the hemodynamic response exhibits a lag of about 4–6 s [Rosen et al., 1998] and regarding that a 3‐s interval (2.5 s picture presentation plus 0.5 s instruction) preceded the regulation phase regulatory effects are expected to start between 7–9 s after picture onset (zero on the time‐axis represents trial‐start). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.\]
Figure 5
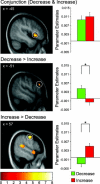
Prefrontal activation peaks and respective parameter estimates. Parameter estimates on the right side stem from the highest activated voxel in the cluster surrounded by the circle. The top panel shows that the OFC exhibits significant overlap of activation across downregulation and upregulation (as revealed by conjunction analysis: conjunction [(increase‐minus‐view) (decrease‐minus‐view)]). The middle panel shows a region in left DLPFC that was significantly stronger activated for downregulation than for upregulation (as revealed by inclusive masking of the decrease‐minus‐increase contrast with the decrease‐minus‐view contrast). The bottom panel shows a region in right DLPFC that was significantly stronger activated for upregulation than for downregulation (as revealed by inclusive masking of the increase‐minus‐decrease contrast with the increase‐minus‐view contrast). Activations are overlaid on subjects' mean anatomy at a level of P < 0.001 uncorrected; the color scale denotes _t_‐values. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.\]
Figure 6
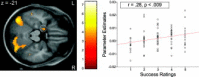
Correlation between left amygdala activation and trial‐by‐trial success ratings in the increase condition. On the left side, a horizontal section displays left amygdala activity that correlated positively with subjects' ratings of regulation success during scanning. Activations are overlaid on subjects' mean anatomy at a level of P < 0.001 uncorrected; the color scale denotes _t_‐values. For the regression plot on the right side data were extracted from a 6‐mm sphere around the highest activated voxel in the amygdala (denoted by the circle). Each data point represents one subject's mean hemodynamic response over trials with the same success rating. Note that not all categories were used by all subjects (i.e., some subjects did not use the rating “2,” others never rated their success above “4,” etc.). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.\]
Similar articles
- Emotion regulation in spider phobia: role of the medial prefrontal cortex.
Hermann A, Schäfer A, Walter B, Stark R, Vaitl D, Schienle A. Hermann A, et al. Soc Cogn Affect Neurosci. 2009 Sep;4(3):257-67. doi: 10.1093/scan/nsp013. Epub 2009 Apr 27. Soc Cogn Affect Neurosci. 2009. PMID: 19398537 Free PMC article. - Association of neural and physiological responses during voluntary emotion suppression.
Ohira H, Nomura M, Ichikawa N, Isowa T, Iidaka T, Sato A, Fukuyama S, Nakajima T, Yamada J. Ohira H, et al. Neuroimage. 2006 Feb 1;29(3):721-33. doi: 10.1016/j.neuroimage.2005.08.047. Epub 2005 Oct 24. Neuroimage. 2006. PMID: 16249100 Clinical Trial. - Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression.
Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Johnstone T, et al. J Neurosci. 2007 Aug 15;27(33):8877-84. doi: 10.1523/JNEUROSCI.2063-07.2007. J Neurosci. 2007. PMID: 17699669 Free PMC article. - Neuroimaging cognitive reappraisal in clinical populations to define neural targets for enhancing emotion regulation. A systematic review.
Zilverstand A, Parvaz MA, Goldstein RZ. Zilverstand A, et al. Neuroimage. 2017 May 1;151:105-116. doi: 10.1016/j.neuroimage.2016.06.009. Epub 2016 Jun 8. Neuroimage. 2017. PMID: 27288319 Free PMC article. Review. - Neural underpinnings of individual differences in emotion regulation: A systematic review.
Morawetz C, Basten U. Morawetz C, et al. Neurosci Biobehav Rev. 2024 Jul;162:105727. doi: 10.1016/j.neubiorev.2024.105727. Epub 2024 May 15. Neurosci Biobehav Rev. 2024. PMID: 38759742 Review.
Cited by
- Transcranial Direct Current Stimulation (tDCS) of the Right Inferior Frontal Gyrus Attenuates Skin Conductance Responses to Unpredictable Threat Conditions.
Herrmann MJ, Beier JS, Simons B, Polak T. Herrmann MJ, et al. Front Hum Neurosci. 2016 Jul 12;10:352. doi: 10.3389/fnhum.2016.00352. eCollection 2016. Front Hum Neurosci. 2016. PMID: 27462211 Free PMC article. - Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies.
Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, Weber J, Ochsner KN. Buhle JT, et al. Cereb Cortex. 2014 Nov;24(11):2981-90. doi: 10.1093/cercor/bht154. Epub 2013 Jun 13. Cereb Cortex. 2014. PMID: 23765157 Free PMC article. - Fear conditioning and early life vulnerabilities: two distinct pathways of emotional dysregulation and brain dysfunction in PTSD.
Lanius RA, Frewen PA, Vermetten E, Yehuda R. Lanius RA, et al. Eur J Psychotraumatol. 2010;1. doi: 10.3402/ejpt.v1i0.5467. Epub 2010 Dec 10. Eur J Psychotraumatol. 2010. PMID: 22893793 Free PMC article. - Mindfulness meditation-based pain relief: a mechanistic account.
Zeidan F, Vago DR. Zeidan F, et al. Ann N Y Acad Sci. 2016 Jun;1373(1):114-27. doi: 10.1111/nyas.13153. Ann N Y Acad Sci. 2016. PMID: 27398643 Free PMC article. Review. - Real-time fMRI feedback training may improve chronic tinnitus.
Haller S, Birbaumer N, Veit R. Haller S, et al. Eur Radiol. 2010 Mar;20(3):696-703. doi: 10.1007/s00330-009-1595-z. Epub 2009 Sep 16. Eur Radiol. 2010. PMID: 19760238
References
- Adcock RA, Lutomski K, McLeod SR, Soneji DJ, Gabriele JDE ( 2005): Real‐time fMRI during the psychotherapy session: toward a methodology to augment therapeutic benefit, exemplary data. Abstract Presented at the Human Brain Mapping Conference 2005, Toronto, Canada.
- Anand A, Li Y, Wang Y, Wu J, Gao S, Bukhari L, Mathews VP, Kalnin A, Lowe MJ ( 2005): Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study. Biol Psychiatry 57: 1079–1088. - PubMed
- Anders S, Weiskopf N, Lule D, Birbaumer N ( 2004a): Infrared oculography—validation of a new method to monitor startle eyeblink amplitudes during fMRI. Neuroimage 22: 767–770. - PubMed
- Anderson AK, Christoff K, Stappen I, Panitz D, Ghahremani DG, Glover G, Gabrieli JD, Sobel N ( 2003): Dissociated neural representations of intensity and valence in human olfaction. Nat Neurosci 6: 196–202. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources