Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning - PubMed (original) (raw)
Comparative Study
Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning
Ann E Wilensky et al. J Neurosci. 2006.
Abstract
In the standard model of pavlovian fear learning, sensory input from neutral and aversive stimuli converge in the lateral nucleus of the amygdala (LA), in which alterations in synaptic transmission encode the association. During fear expression, the LA is thought to engage the central nucleus of the amygdala (CE), which serves as the principal output nucleus for the expression of conditioned fear responses. In the present study, we reexamined the roles of LA and CE. Specifically, we asked whether CE, like LA, might also be involved in fear learning and memory consolidation. Using functional inactivation methods, we first show that CE is involved not only in the expression but also the acquisition of fear conditioning. Next, we show that inhibition of protein synthesis in CE after training impairs fear memory consolidation. These findings indicate that CE is not only involved in fear expression but, like LA, is also involved in the learning and consolidation of pavlovian fear conditioning.
Figures
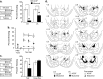
Figure 1.
The role of CE in the acquisition of auditory fear conditioning. a, Procedural outline and mean ± SE percentage freezing during CS presentation averaged across all tone trials. Freezing scores after functional inactivation of either CE or LA were significantly decreased from control groups (*p < 0.05). The average freezing after functional inactivation of CE was also significantly different from that of LA (p < 0.05). b, Mean ± SE percentage freezing per group during each tone trial. c, Procedural outline for functional inactivation of CE during expression and mean ± SE percentage freezing to CS presentation averaged across all tone trials during testing and 24 h later, drug free. Although muscimol significantly decreased freezing (*p < 0.05), muscimol-treated animals did not differ from controls when retested drug free. d, Cannula tip placements for all animals included in analysis (drawings adapted from Paxinos and Watson, 1997). Numbers on the left refer to posterior to bregma in millimeters. B, Basal nucleus.
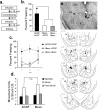
Figure 2.
State-dependent and shock reactivity tests. a, Experimental protocol. b, Mean ± SE percentage freezing for each group. *p < 0.05 relative to the ACSF–Musc and Musc–Musc groups. c, Mean ± SE percentage freezing for each group across each trial. d, Mean ± SE movement units before, during, and after the shock US. e, Top, Photomicrograph of a representative cannula placement in the CE. Bottom, Cannula tip placements for all animals included in analysis (drawings adapted from Paxinos and Watson, 1997). Numbers on the left refer to posterior to bregma in millimeters. B, Basal nucleus.
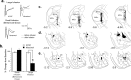
Figure 3.
Evoked activity in LA after functional inactivation of CE. a, Examples of averaged evoked field potentials before and after infusions. Large infusions of muscimol produced a significant reduction in field potential amplitude and area, whereas small infusions had little effect. b, Ratio of the amplitude of the negative-going component after infusions to the baseline amplitude. After large infusions of muscimol designed to spread to LA, the amplitude is observed to be significantly decreased relative to ACSF-infused animals (*p < 0.001). However, after infusion of the smaller dose of muscimol used in our behavioral experiments, the field responses in muscimol-infused rats did not differ from that of ACSF-infused rats. c, Thalamic electrode placements for all animals included in analysis. MGd, Dorsal division of the medial geniculate nucleus; MGv, ventral division of the medial geniculate nucleus; M, medial division of the medial geniculate nucleus; S, suprageniculate nucleus; P, posterior intralaminar nucleus. d, LA recording electrode placements. e, CE cannula placements.
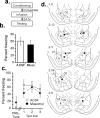
Figure 4.
Posttraining functional inactivation of CE. a, Procedural outline. b, Mean ± SE percentage freezing during CS tests averaged across all tone trials. Animals infused with muscimol did not significantly differ from controls. c, Mean ± SE percentage freezing per group during each tone trial. No significant differences were observed in freezing to context or during any tone trial. d, CE cannula placements for all animals included in analysis. B, Basal nucleus.
Figure 5.
The role of CE in the consolidation of auditory fear conditioning. a, Procedural outline. b, STM mean ± SE percentage freezing averaged across all CS presentations. Anisomycin-treated (Aniso) and ACSF-infused animals did not significantly differ. c, LTM mean ± SE percentage freezing averaged across all CS presentations. Intra-CE infusion of anisomycin significantly impaired LTM (*p < 0.001). In contrast, after retraining, animals froze at the same levels as controls. d, Mean ± SE percentage freezing per group during each LTM test CS. All three trials show the same pattern of significance as in c. e, The LTM to STM ratios show that consolidation is significantly impaired after infusions of anisomycin into CE. *p < 0.01. f, g, Mean ± SE percentage freezing during the STM and LTM tests in rats that received intra-PRh infusion of vehicle or anisomycin. h, CE and PRh cannula placements for all animals included in analysis. B, Basal nucleus.
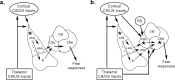
Figure 6.
A revised model of the role of the amygdala in fear conditioning. a, Original model. CS and US information travels through the thalamic and cortical pathways to converge in the dorsal LA. The information is then encoded in LA. During expression, sensory CS information is transmitted from LA to CE, both directly and by way of the adjacent basal nucleus (B). The CE is the major source of output pathways for fear responses such as freezing. b, A new possibility. CS and US information are transmitted through multiple pathways to both the LA and CE, resulting in plasticity and memory formation that is distributed within each of the nuclei. Auditory and somatosensory information originating in the thalamus may reach CEm, the principle source of brainstem-projecting outputs from the CE, either directly or via the adjacent CEl. In addition, projections from the LA may reach the CEm either via the CEl or via projections from the intercalated cell masses, which lie between the LA and CE. Convergence of sensory inputs in CEm results in synaptic plasticity and serves as an additional site of fear memory formation. It remains unclear, however, whether plasticity and memory formation in CE occurs in parallel to that in LA or relies on distributed plasticity throughout the amygdala that is initiated by LA neurons. For additional details, see Paré et al. (2004). Asterisks indicate site(s) of presumed synaptic plasticity/learning. LAd, Dorsal division of the LA; LAm, medial division of the LA; PB, peribrachial nucleus.
Comment in
- The central amygdala joins the lateral amygdala in the fear memory party.
Miller SS, Urcelay GP. Miller SS, et al. J Neurosci. 2007 Feb 28;27(9):2151-2. doi: 10.1523/JNEUROSCI.5460-06.2007. J Neurosci. 2007. PMID: 17329410 Free PMC article. No abstract available.
Similar articles
- New vistas on amygdala networks in conditioned fear.
Paré D, Quirk GJ, Ledoux JE. Paré D, et al. J Neurophysiol. 2004 Jul;92(1):1-9. doi: 10.1152/jn.00153.2004. J Neurophysiol. 2004. PMID: 15212433 Review. - Activation of ERK/MAP kinase in the amygdala is required for memory consolidation of pavlovian fear conditioning.
Schafe GE, Atkins CM, Swank MW, Bauer EP, Sweatt JD, LeDoux JE. Schafe GE, et al. J Neurosci. 2000 Nov 1;20(21):8177-87. doi: 10.1523/JNEUROSCI.20-21-08177.2000. J Neurosci. 2000. PMID: 11050141 Free PMC article. - An organization of visual and auditory fear conditioning in the lateral amygdala.
Bergstrom HC, Johnson LR. Bergstrom HC, et al. Neurobiol Learn Mem. 2014 Dec;116:1-13. doi: 10.1016/j.nlm.2014.07.008. Epub 2014 Jul 27. Neurobiol Learn Mem. 2014. PMID: 25076183 - Sidman instrumental avoidance initially depends on lateral and basal amygdala and is constrained by central amygdala-mediated Pavlovian processes.
Lázaro-Muñoz G, LeDoux JE, Cain CK. Lázaro-Muñoz G, et al. Biol Psychiatry. 2010 Jun 15;67(12):1120-7. doi: 10.1016/j.biopsych.2009.12.002. Epub 2010 Jan 27. Biol Psychiatry. 2010. PMID: 20110085 Free PMC article. - Molecular mechanisms of threat learning in the lateral nucleus of the amygdala.
Sears RM, Schiff HC, LeDoux JE. Sears RM, et al. Prog Mol Biol Transl Sci. 2014;122:263-304. doi: 10.1016/B978-0-12-420170-5.00010-6. Prog Mol Biol Transl Sci. 2014. PMID: 24484705 Review.
Cited by
- Oxytocin excites BNST interneurons and inhibits BNST output neurons to the central amygdala.
Francesconi W, Berton F, Olivera-Pasilio V, Dabrowska J. Francesconi W, et al. Neuropharmacology. 2021 Jul 1;192:108601. doi: 10.1016/j.neuropharm.2021.108601. Epub 2021 May 7. Neuropharmacology. 2021. PMID: 33971215 Free PMC article. - A role for the extended amygdala in the fear-enhancing effects of acute selective serotonin reuptake inhibitor treatment.
Ravinder S, Burghardt NS, Brodsky R, Bauer EP, Chattarji S. Ravinder S, et al. Transl Psychiatry. 2013 Jan 15;3(1):e209. doi: 10.1038/tp.2012.137. Transl Psychiatry. 2013. PMID: 23321806 Free PMC article. - Divergent input patterns to the central lateral amygdala play a duet in fear memory formation.
Gao JH, Liu YY, Xu HX, Wu K, Zhang LL, Cheng P, Peng XH, Cao JL, Hua R, Zhang YM. Gao JH, et al. iScience. 2024 Sep 4;27(10):110886. doi: 10.1016/j.isci.2024.110886. eCollection 2024 Oct 18. iScience. 2024. PMID: 39319272 Free PMC article. - Suppression of conditioning to ambiguous cues by pharmacogenetic inhibition of the dentate gyrus.
Tsetsenis T, Ma XH, Lo Iacono L, Beck SG, Gross C. Tsetsenis T, et al. Nat Neurosci. 2007 Jul;10(7):896-902. doi: 10.1038/nn1919. Epub 2007 Jun 3. Nat Neurosci. 2007. PMID: 17558402 Free PMC article. - Mechanisms of fear learning and extinction: synaptic plasticity-fear memory connection.
Luchkina NV, Bolshakov VY. Luchkina NV, et al. Psychopharmacology (Berl). 2019 Jan;236(1):163-182. doi: 10.1007/s00213-018-5104-4. Epub 2018 Nov 10. Psychopharmacology (Berl). 2019. PMID: 30415278 Free PMC article. Review.
References
- Alberini CM, Ghirardi M, Huang YY, Nguyen PV, Kandel ER. A molecular switch for the consolidation of long-term memory: cAMP-inducible gene expression. Ann NY Acad Sci. 1995;758:261–286. - PubMed
- Amorapanth P, LeDoux JE, Nader K. Different lateral amygdala outputs mediate reactions and actions elicited by a fear-arousing stimulus. Nat Neurosci. 2000;3:74–79. - PubMed
- Applegate CD, Frysinger RC, Kapp BS, Gallagher M. Multiple unit activity recorded from amygdala central nucleus during Pavlovian heart rate conditioning in rabbit. Brain Res. 1982;238:457–462. - PubMed
- Applegate CD, Kapp BS, Underwood MD, McNall CL. Autonomic and somatomotor effects of amygdala central N. stimulation in awake rabbits. Physiol Behav. 1983;31:353–360. - PubMed
Publication types
MeSH terms
Grants and funding
- R37 MH38774/MH/NIMH NIH HHS/United States
- K05 MH067048/MH/NIMH NIH HHS/United States
- R01 MH046516/MH/NIMH NIH HHS/United States
- R01 MH46516/MH/NIMH NIH HHS/United States
- R37 MH038774/MH/NIMH NIH HHS/United States
- F31 MH63612/MH/NIMH NIH HHS/United States
- F31 MH063612/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources