Monkey dorsolateral prefrontal cortex sends task-selective signals directly to the superior colliculus - PubMed (original) (raw)
Comparative Study
Monkey dorsolateral prefrontal cortex sends task-selective signals directly to the superior colliculus
Kevin Johnston et al. J Neurosci. 2006.
Abstract
The dorsolateral prefrontal cortex (DLPFC) has been implicated in the ability to perform complex behaviors requiring the implementation of cognitive control. A central supposition of models of prefrontal function is that the DLPFC engages control by selectively modulating the activity of target structures to which it is connected, but no studies in the primate have directly investigated DLPFC output signals. Here, we recorded the activity of DLPFC neurons identified as sending a direct projection to the superior colliculus, a midbrain oculomotor structure, while monkeys performed alternating blocks of trials in which they had to look toward a flashed peripheral stimulus (prosaccades) and trials in which they had to look away from the stimulus in the opposite direction (antisaccades). We report the first direct evidence that the primate DLPFC sends task-selective signals to a target structure. This supports the notion that the DLPFC orchestrates the activity of other brain areas in accordance with task requirements.
Figures
Figure 1.
Experimental task. Each trial began with the presentation of a fixation point at the center of the screen, which the monkey was required to fixate. A visual stimulus then appeared to the right or left of fixation. Monkeys were required to make either a prosaccade or antisaccade (arrows) depending on the task rule in effect. After 30 correct responses, the task rule switched without explicit signal to the animals.
Figure 2.
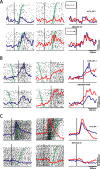
Recording locations and schematic representation of antidromic identification technique. A, Recording locations in monkeys R and W, reconstructed from MRI images. Slices are separated by 1 mm. Blue dots represent locations of neurons showing significant effect of stimulus location, red dots indicate locations of neurons showing significant effect of saccade direction, and green dots indicate locations of neurons showing a significant interaction between stimulus location and saccade direction. iar, Inferior arcuate sulcus; ps, principal sulcus; sar, superior arcuate sulcus. A, Anterior; L, lateral; M, medial; P, posterior. B, Right, schematic representation of experimental method for antidromic activation. Left, Waveforms depicting activity recorded in the DLPFC showing artifact caused by stimulation and stimulation-elicited action potential (AP). C, Right, Schematic representation of collision test. Left, Activation waveforms depicting DLPFC activity during collision test. Spontaneous action potential (AP) triggers stimulation pulse to SC. After collision, no action potential is observed in DLPFC neuron. D, Antidromic latencies. No difference in latency was found between neurons showing significant effects and those showing no effects (ANOVA, p > 0.05).
Figure 3.
Neural activity during the preparatory period. A, Mean activity during the 500 ms immediately preceding presentation of the peripheral stimulus for antisaccade trials (ordinate) and prosaccade trials (abscissa). Solid line indicates least-squares fit of data points, and dashed line represents unity. B, Pro/anti contrast ratios for the population of corticotectal neurons recorded. Ratios were calculated using mean activity in the preparatory period described above. Black bars represent neurons with ratio values significantly greater than zero. Three additional neurons not shown have ratio values above 0.5.
Figure 4.
Single neuron examples. Red lines depict activity on antisaccade trials, and blue lines prosaccade trials. A, Neuron showing a significant effect of stimulus location. Functions and rasters are aligned on stimulus onset. Green circles represent SRTs. Trials are sorted by reaction time. Top, Activity for conditions in which stimulus is presented on the contralateral side (contralateral prosaccades, ipsilateral antisaccades). Bottom, Activity for conditions in which stimulus is presented on the ipsilateral side (ipsilateral prosaccades, contralateral antisaccades). Neuron was more active for ipsilateral stimuli regardless of saccade direction or task rule. B, Neuron showing significant effect of saccade direction. Spike density functions and rasters are aligned on saccade onset. Green circles represent time of stimulus presentation. Top, Activity for contralateral prosaccades and ipsilateral antisaccades, as in A. Bottom, Also same as A. Neuron was more active for contralateral saccades regardless of stimulus location or task rule. C, Neuron showing an interaction between stimulus location and task rule. Functions and rasters are aligned on stimulus onset. Green circles represent SRT. Activity depicted in top and bottom as in A and B. Neuron was more active for condition in which stimulus was presented on the contralateral side and more active for antisaccades than prosaccades in this condition. All spike density functions were constructed by convolving spike trains with a 30 ms Gaussian activation function. Statistical significance for all neurons was evaluated by ANOVA at p < 0.05.
Figure 5.
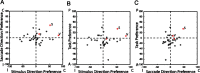
Plots of stimulus, saccade, and task indices for all neurons showing significant effects on ANOVA. Black dots, DLPFC neurons. Red dots, Neurons previously recorded from the SC (Everling and Munoz, 2000, their Fig. 4). P, Prosaccade; A, antisaccade; C, contralateral; I, ipsilateral; S, Saccade; V, visual; F, fixation. A, Saccade direction index versus stimulus direction index. B, Task index versus stimulus direction index. C, Task index versus saccade direction index.
Figure 6.
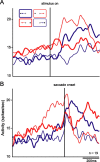
Spike density functions for the population of neurons showing significant interaction on ANOVA. Red lines represent activity on antisaccade trials, and blue lines indicate activity on prosaccade trials. Thick and thin lines represent activity for saccades directed to the side ipsilateral and contralateral to the recorded (right) hemisphere respectively. A, Functions aligned on stimulus onset. B, Functions aligned on saccade onset. More activity is present on antisaccade trials than prosaccade trials. More specifically, an elevated response is observed before the saccade for trials on which the visual stimulus is presented on the contralateral side and saccades are directed toward the ipsilateral side (i.e., contraversive antisaccades).
Figure 7.
Histograms depicting correlations between neural activity and SRT for the population of antidromic neurons for ipsilateral (ipsi) and contralateral (contra) prosaccades and antisaccades. Correlations were computed between mean activity for each condition in an 80 ms window immediately after presentation of the visual stimulus and the SRT for the corresponding condition. Neural activity was correlated with SRT for both ipsilateral and contralateral antisaccades. A, Correlations for ipsilateral prosaccades. B, Correlations for contralateral prosaccades. C, Correlations for contralateral antisaccades. D, Correlations for ipsilateral antisaccades. No significant correlations were found for prosaccades (t tests, evaluated at p < 0.05). Neurons showing a significant interaction effect are highlighted in black.
Figure 8.
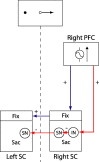
Possible mechanism by which enhanced DLPFC signal for contralateral antisaccade targets could facilitate antisaccade task performance via the SC. Excitatory descending signal could enhance activity of fixation (Fix) neurons in the ipsilateral (right) rostral SC and, in turn, the contralateral (left) SC. Alternatively, excitatory descending signal could be sent to an inhibitory interneuron (IN) in the caudal saccade zone of the SC (Sac), which would in turn inhibit saccade neurons (SN), leading to decreased probability of triggering a reflexive saccade toward the stimulus. This would also lead to decreased inhibition of saccade neurons in the contralateral SC, which would facilitate execution of a rightward saccade.
Similar articles
- Task-relevant output signals are sent from monkey dorsolateral prefrontal cortex to the superior colliculus during a visuospatial working memory task.
Johnston K, Everling S. Johnston K, et al. J Cogn Neurosci. 2009 May;21(5):1023-38. doi: 10.1162/jocn.2009.21067. J Cogn Neurosci. 2009. PMID: 18702590 - Prefrontal cortex deactivation in macaques alters activity in the superior colliculus and impairs voluntary control of saccades.
Koval MJ, Lomber SG, Everling S. Koval MJ, et al. J Neurosci. 2011 Jun 8;31(23):8659-68. doi: 10.1523/JNEUROSCI.1258-11.2011. J Neurosci. 2011. PMID: 21653870 Free PMC article. - Macaque dorsolateral prefrontal cortex does not suppress saccade-related activity in the superior colliculus.
Johnston K, Koval MJ, Lomber SG, Everling S. Johnston K, et al. Cereb Cortex. 2014 May;24(5):1373-88. doi: 10.1093/cercor/bhs424. Epub 2013 Jan 10. Cereb Cortex. 2014. PMID: 23307633 - Prefrontal cortex and neural mechanisms of executive function.
Funahashi S, Andreau JM. Funahashi S, et al. J Physiol Paris. 2013 Dec;107(6):471-82. doi: 10.1016/j.jphysparis.2013.05.001. Epub 2013 May 15. J Physiol Paris. 2013. PMID: 23684970 Review. - Control of the superior colliculus by the lateral prefrontal cortex.
Everling S, Johnston K. Everling S, et al. Philos Trans R Soc Lond B Biol Sci. 2013 Sep 9;368(1628):20130068. doi: 10.1098/rstb.2013.0068. Print 2013 Oct 19. Philos Trans R Soc Lond B Biol Sci. 2013. PMID: 24018729 Free PMC article. Review.
Cited by
- Conflict Resolution as Near-Threshold Decision-Making: A Spiking Neural Circuit Model with Two-Stage Competition for Antisaccadic Task.
Lo CC, Wang XJ. Lo CC, et al. PLoS Comput Biol. 2016 Aug 23;12(8):e1005081. doi: 10.1371/journal.pcbi.1005081. eCollection 2016 Aug. PLoS Comput Biol. 2016. PMID: 27551824 Free PMC article. - Neuronal representation of task performance in the medial frontal cortex undergoes dynamic alterations dependent upon the demand for volitional control of action.
Matsuzaka Y, Akiyama T, Mushiake H. Matsuzaka Y, et al. Exp Brain Res. 2013 Sep;229(3):395-405. doi: 10.1007/s00221-013-3454-z. Epub 2013 Mar 12. Exp Brain Res. 2013. PMID: 23479139 - Roles of the primate motor thalamus in the generation of antisaccades.
Kunimatsu J, Tanaka M. Kunimatsu J, et al. J Neurosci. 2010 Apr 7;30(14):5108-17. doi: 10.1523/JNEUROSCI.0406-10.2010. J Neurosci. 2010. PMID: 20371831 Free PMC article. - Age-related changes in pupil dynamics and task modulation across the healthy lifespan.
Huang J, Smorenburg ML, Yep R, Riek HC, Calancie OG, Kirkpatrick RH, Brien DC, Coe BC, Wang CA, Munoz DP. Huang J, et al. Front Neurosci. 2024 Nov 19;18:1445727. doi: 10.3389/fnins.2024.1445727. eCollection 2024. Front Neurosci. 2024. PMID: 39628657 Free PMC article. - Ketamine Alters Outcome-Related Local Field Potentials in Monkey Prefrontal Cortex.
Skoblenick KJ, Womelsdorf T, Everling S. Skoblenick KJ, et al. Cereb Cortex. 2016 Jun;26(6):2743-2752. doi: 10.1093/cercor/bhv128. Epub 2015 Jun 3. Cereb Cortex. 2016. PMID: 26045564 Free PMC article.
References
- Asaad WF, Rainer G, Miller EK. Task-specific neural activity in the primate prefrontal cortex. J Neurophysiol. 2000;84:451–459. - PubMed
- Chafee MV, Goldman-Rakic PS. Inactivation of parietal and prefrontal cortex reveals interdependence of neural activity during memory-guided saccades. J Neurophysiol. 2000;83:1550–1556. - PubMed
- Condy C, Wattiez N, Rivaud-Pechoux S, Tremblay L, Gaymard B. Antisaccade deficit after inactivation of the principal sulcus in monkeys. Cereb Cortex. 2006 in press. - PubMed
- Creutzfeldt OD. Berlin: Springer; 1983. Cortex cerebri: performance, structural and functional organization of the cortex.
- Curtis CE, D'Esposito M. Success and failure suppressing reflexive behaviour. J Cogn Neurosci. 2003;15:409–418. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources