Amyloid-beta vaccination, but not nitro-nonsteroidal anti-inflammatory drug treatment, increases vascular amyloid and microhemorrhage while both reduce parenchymal amyloid - PubMed (original) (raw)
Amyloid-beta vaccination, but not nitro-nonsteroidal anti-inflammatory drug treatment, increases vascular amyloid and microhemorrhage while both reduce parenchymal amyloid
D M Wilcock et al. Neuroscience. 2007.
Abstract
Vaccination with Abeta(1-42) and treatment with NCX-2216, a novel nitric oxide releasing flurbiprofen derivative, have each been shown separately to reduce amyloid deposition in transgenic mice and have been suggested as potential therapies for Alzheimer's disease. In the current study we treated doubly transgenic amyloid precursor protein and presenilin-1 (APP+PS1) mice with Abeta(1-42) vaccination, NCX-2216 or both drugs simultaneously for 9 months. We found that all treatments reduced amyloid deposition, both compact and diffuse, to the same extent while only vaccinated animals, with or without nonsteroidal anti-inflammatory drug (NSAID) treatment, showed increased microglial activation associated with the remaining amyloid deposits. We also found that active Abeta vaccination resulted in significantly increased cerebral amyloid angiopathy and associated microhemorrhages, while NCX-2216 did not, in spite of similar reductions in parenchymal amyloid. Co-administration of NCX-2216 did not attenuate this effect of the vaccine. This is the first report showing that active immunization can result in increased vascular amyloid and microhemorrhage, as has been observed with passive immunization. Co-administration of an NSAID agent with Abeta vaccination does not substantially modify the effects of Abeta immunotherapy. The difference between these treatments with respect to vascular amyloid development may reflect the clearance-promoting actions of the vaccine as opposed to the production-modifying effects proposed for flurbiprofen.
Figures
Figure 1
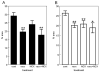
Aβ1–42 vaccination, with or without NCX-2216 treatment, produces detectable anti-Aβ antibody titers. The graph shows antibody titers for mice receiving either no treatment (Cont), Aβ1–42 vaccination (vacc), NCX-2216 diet (NCX) or both Aβ1–42 vaccination with NCX-2216 diet (vacc+NCX). Titers are shown as μg IgG per ml serum after dissociation from circulating Aβ. ** indicates P<0.01.
Figure 2
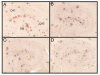
Aβ immunohistochemistry is reduced in all three treatment groups. Aβ immunohistochemistry is shown in the hippocampus for APP+PS1 transgenic mice receiving no treatment (Panel A), Aβ1–42 vaccination (Panel B), NCX-2216 diet (Panel C) or both Aβ1–42 vaccination with NCX-2216 diet (Panel D). In Panel A, CA1: cornu ammonis 1, CA3: cornu ammonis 3, DG: dentate gyrus. Scale bar in Panel A = 120μm.
Figure 3
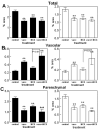
Quantification of Aβ immunohistochemistry reveals reductions in all three treatment groups compared with control treated APP+PS1 transgenic mice. The graphs show percent area occupied with positive stain for mice receiving either no treatment (cont), Aβ1–42 vaccination (vacc), NCX-2216 diet (NCX) or both Aβ1–42 vaccination with NCX-2216 diet (vacc+NCX). Panel A shows quantification in the frontal cortex while panel B shows quantification in the hippocampus. * indicates P<0.05, ** indicates P<0.01 compared to untreated mice.
Figure 4
Congo red staining is reduced in all three treatment groups. Congo red staining is shown in the hippocampus for APP+PS1 transgenic mice receiving no treatment (Panel A), Aβ1–42 vaccination (Panel B), NCX-2216 diet (Panel C) or both Aβ1–42 vaccination with NCX-2216 diet (Panel D). In Panel A CA1: cornu ammonis 1, CA3: cornu ammonis 3, DG: dentate gyrus. Scale bar in Panel A = 120μm.
Figure 5
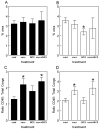
Quantification of Congo red staining reveals reductions in total Congo red in all three treatment groups, but increased vascular Congo red following Aβ1–42 vaccination. The graphs show percent area occupied with positive stain for mice receiving either no treatment (cont), Aβ1–42 vaccination (vacc), NCX-2216 diet (NCX) or both Aβ1–42 vaccination with NCX-2216 diet (vacc+NCX). Solid bars indicate values for the frontal cortex while open bars indicate values for the hippocampus. Panel A shows quantification of total Congo red, panel B shows quantification of vascular Congo red and panel C shows calculation of parenchymal Congo red. ** indicates P<0.01 compared to control and as shown in panel B.
Figure 6
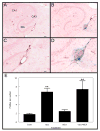
CAA- associated microhemorrhage is increased following Aβ1–42 vaccination. Sections are stained for hemosiderin (extravenous iron) with Prussian blue and cells are counterstained with Texas red. Panels A and C show the hippocampus of a control treated APP+PS1 transgenic mouse while panels B and D show the hippocampus of an Aβ1–42 vaccinated APP+PS1 transgenic mouse. For Panels A and B, the scale bar in panel A represents 120μm. Panels C and D are a higher magnification of the area shown in the box of panels A and B, and the scale bar in panel C = 50μm. In Panel A, CA1: cornu ammonis 1, CA3: cornu ammonis 3, DG: dentate gyrus. Panel E shows quantification of Prussian blue staining and is shown as the number of positive profiles per section for mice receiving either no treatment (cont), Aβ1–42 vaccination (vacc), NCX-2216 diet (NCX) or both Aβ1–42 vaccination with NCX-2216 diet (vacc+NCX). ** indicates P<0.01 compared to the control group.
Figure 7
CD45 immunohistochemistry is increased around remaining plaques in mice administered Aβ1–42 vaccination. CD45 immunohistochemistry counterstained with Congo red is shown in the hippocampus for APP+PS1 transgenic mice receiving no treatment (Panel A), Aβ1–42 vaccination (Panel B), NCX-2216 diet (Panel C) or both Aβ1–42 vaccination with NCX-2216 diet (Panel D). In Panel A CA1: cornu ammonis 1, CA3: cornu ammonis 3, DG: dentate gyrus. Scale bar in Panel A = 120μm.
Figure 8
Quantification of CD45 immunohistochemistry reveals increases around remaining deposits following Aβ1–42 vaccination. The graphs show percent area occupied with positive stain for mice receiving either no treatment (cont), Aβ1–42 vaccination (vacc), NCX-2216 diet (NCX) or both Aβ1–42 vaccination with NCX-2216 diet (vacc+NCX). Panels A and B show quantification of total percent area occupied with positive stain for CD45 while panels C and D show calculated ratios of CD45 staining to Congo red. Panels A and C show quantification in the frontal cortex while panels B and D show quantification in the hippocampus. * indicates P<0.05 compared to control APP+PS1 mice.
Similar articles
- Mannan-Abeta28 conjugate prevents Abeta-plaque deposition, but increases microhemorrhages in the brains of vaccinated Tg2576 (APPsw) mice.
Petrushina I, Ghochikyan A, Mkrtichyan M, Mamikonyan G, Movsesyan N, Ajdari R, Vasilevko V, Karapetyan A, Lees A, Agadjanyan MG, Cribbs DH. Petrushina I, et al. J Neuroinflammation. 2008 Sep 29;5:42. doi: 10.1186/1742-2094-5-42. J Neuroinflammation. 2008. PMID: 18823564 Free PMC article. - Microglial activation and beta -amyloid deposit reduction caused by a nitric oxide-releasing nonsteroidal anti-inflammatory drug in amyloid precursor protein plus presenilin-1 transgenic mice.
Jantzen PT, Connor KE, DiCarlo G, Wenk GL, Wallace JL, Rojiani AM, Coppola D, Morgan D, Gordon MN. Jantzen PT, et al. J Neurosci. 2002 Mar 15;22(6):2246-54. doi: 10.1523/JNEUROSCI.22-06-02246.2002. J Neurosci. 2002. PMID: 11896164 Free PMC article. - Immunotherapy reduces vascular amyloid-beta in PDAPP mice.
Schroeter S, Khan K, Barbour R, Doan M, Chen M, Guido T, Gill D, Basi G, Schenk D, Seubert P, Games D. Schroeter S, et al. J Neurosci. 2008 Jul 2;28(27):6787-93. doi: 10.1523/JNEUROSCI.2377-07.2008. J Neurosci. 2008. PMID: 18596154 Free PMC article. - Antibody responses, amyloid-beta peptide remnants and clinical effects of AN-1792 immunization in patients with AD in an interrupted trial.
Kokjohn TA, Roher AE. Kokjohn TA, et al. CNS Neurol Disord Drug Targets. 2009 Apr;8(2):88-97. doi: 10.2174/187152709787847315. CNS Neurol Disord Drug Targets. 2009. PMID: 19355930 Free PMC article. Review. - Amyloid-beta immunotherapy for Alzheimer's disease.
Fu HJ, Liu B, Frost JL, Lemere CA. Fu HJ, et al. CNS Neurol Disord Drug Targets. 2010 Apr;9(2):197-206. doi: 10.2174/187152710791012017. CNS Neurol Disord Drug Targets. 2010. PMID: 20205640 Free PMC article. Review.
Cited by
- Amyloid beta immunization worsens iron deposits in the choroid plexus and cerebral microbleeds.
Joseph-Mathurin N, Dorieux O, Trouche SG, Boutajangout A, Kraska A, Fontès P, Verdier JM, Sigurdsson EM, Mestre-Francés N, Dhenain M. Joseph-Mathurin N, et al. Neurobiol Aging. 2013 Nov;34(11):2613-22. doi: 10.1016/j.neurobiolaging.2013.05.013. Epub 2013 Jun 22. Neurobiol Aging. 2013. PMID: 23796662 Free PMC article. - Chronological age impacts immunotherapy and monocyte uptake independent of amyloid load.
Li Q, Lebson L, Lee DC, Nash K, Grimm J, Rosenthal A, Selenica ML, Morgan D, Gordon MN. Li Q, et al. J Neuroimmune Pharmacol. 2012 Mar;7(1):202-14. doi: 10.1007/s11481-011-9329-9. Epub 2011 Dec 27. J Neuroimmune Pharmacol. 2012. PMID: 22198698 - Induction of toll-like receptor 9 signaling as a method for ameliorating Alzheimer's disease-related pathology.
Scholtzova H, Kascsak RJ, Bates KA, Boutajangout A, Kerr DJ, Meeker HC, Mehta PD, Spinner DS, Wisniewski T. Scholtzova H, et al. J Neurosci. 2009 Feb 11;29(6):1846-54. doi: 10.1523/JNEUROSCI.5715-08.2009. J Neurosci. 2009. PMID: 19211891 Free PMC article. - Mannan-Abeta28 conjugate prevents Abeta-plaque deposition, but increases microhemorrhages in the brains of vaccinated Tg2576 (APPsw) mice.
Petrushina I, Ghochikyan A, Mkrtichyan M, Mamikonyan G, Movsesyan N, Ajdari R, Vasilevko V, Karapetyan A, Lees A, Agadjanyan MG, Cribbs DH. Petrushina I, et al. J Neuroinflammation. 2008 Sep 29;5:42. doi: 10.1186/1742-2094-5-42. J Neuroinflammation. 2008. PMID: 18823564 Free PMC article. - Reducing AD-like pathology in 3xTg-AD mouse model by DNA epitope vaccine - a novel immunotherapeutic strategy.
Movsesyan N, Ghochikyan A, Mkrtichyan M, Petrushina I, Davtyan H, Olkhanud PB, Head E, Biragyn A, Cribbs DH, Agadjanyan MG. Movsesyan N, et al. PLoS One. 2008 May 7;3(5):e2124. doi: 10.1371/journal.pone.0002124. PLoS One. 2008. PMID: 18461171 Free PMC article.
References
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O’Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyama I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000;21:383–421. - PMC - PubMed
- Attems J. Sporadic cerebral amyloid angiopathy: pathology, clinical implications, and possible pathomechanisms. Acta Neuropathol. 2005;110:345–359. - PubMed
- Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, Seubert P, Schenk D, Yednock T. Peripherally administered antibodies against amyloid β-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer's disease. Nat Med. 2000;6:916–919. - PubMed
- Bard F, Barbour R, Cannon C, Carretto R, Fox M, Games D, Guido T, Hoenow K, Hu K, Johnson-Wood K, Khan K, Kholodenko D, Lee C, Lee M, Motter R, Nguyen M, Reed A, Schenk D, Tang P, Vasquez N, Seubert P, Yednock T. Epitope and isotype specificities of antibodies to beta -amyloid peptide for protection against Alzheimer's disease-like neuropathology. Proc Natl Acad Sci USA. 2003;100:2023–2028. - PMC - PubMed
- Bernado A, Gasparini L, Ongini E, Minghetti L. Dynamic regulation of microglial functions by the non-steroidal anti-inflammatory drug NCX 2216: Implications for chronic treatments of neurodegenerative diseases. Neurobiol Dis. 2006;22:25–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AG 18478/AG/NIA NIH HHS/United States
- AG 15490/AG/NIA NIH HHS/United States
- AG 25711/AG/NIA NIH HHS/United States
- R01 AG018478/AG/NIA NIH HHS/United States
- P50 AG025711/AG/NIA NIH HHS/United States
- R01 AG015490/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases