Optimal determination of heart tissue 26S-proteasome activity requires maximal stimulating ATP concentrations - PubMed (original) (raw)
Optimal determination of heart tissue 26S-proteasome activity requires maximal stimulating ATP concentrations
Saul R Powell et al. J Mol Cell Cardiol. 2007 Jan.
Abstract
The ubiquitin-proteasome system has been implicated in both cardiac physiology and pathophysiology. Research in this area has been hampered by the lack of a simple, reproducible method to assess 26S-proteasome peptidase activities. The current report demonstrates that one reason for lack of reproducibility is the myriad of ATP concentrations, many of them excessive, which have been used to stimulate peptidase activity. The chymotrypsin-like or caspase-like activities of 26S-proteasome in cardiac tissue isolates were determined using Suc-LLVY-AMC or Z-LLE-AMC, respectively, over a range of ATP concentrations up to 2 mmol/L. The optimal ATP concentration to assess both peptidase activities was found to be in the low micromolar range (from 6 to 100 micromol/L) depending on the cardiac tissue isolate protein (10 to 90 microg protein) contained in the reaction. Increasing ATP beyond the optimal range was inhibitory. In general, chymotrypsin-like and caspase-like activities could be stimulated 2- to 2.5-fold and 1.4- to 1.8-fold, respectively, over basal (ATP, 0 micromol/L), and could be effectively inhibited with lactacystin or Z-Pro-Nle-Asp-CHO, respectively. Based on these observations, an optimized method is presented for ex vivo determination of cardiac 26S-proteasome peptidase activities which was used to confirm inactivation of this complex by myocardial ischemia and reperfusion.
Figures
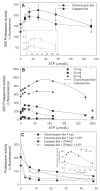
Figure 1. Optimal conditions for determination of cardiac 26S-proteasome activity. Panel A: The effect of ATP concentration
Aliquots (30 μg protein) of rat heart tissue cytosol was assayed for chymotrypsin-like and caspase-like activities in the presence of increasing concentrations of ATP. The inset shows the data normalized to the 0 μmol/L ATP or basal activity to determine magnitude of activation. The values represent the mean ± SEM of 5 to 7 determinations. Panel B: The effect of increasing protein concentrations. Aliquots of rat heart tissue cytosol containing up to 90 μg protein were assayed for chymotrypsin-like and caspase-like activities in the presence of increasing concentrations of ATP. The values represent the mean of 2 to 3 determinations. Panel C: The effect of proteasome inhibitors. Aliquots (30 μg protein) of rat heart tissue cytosol was assayed for chymotrypsin-like and caspase-like activities at the optimal ATP concentration of 28 μmol/L and in the presence of increasing concentrations of the proteasome inhibitors, lactacystin (Lac) and Z-Pro-Nle-Asp-CHO (ZPNAC). The inset shows the effect of increasing ATP concentrations on chymotrypsin-like activity in the absence and presence of Lac, 10 μmol/L. The values represent the mean of 2 to 3 determinations.
Figure 2. Inhibition of 26S-Proteasome peptidase activity by ischemia and reperfusion
Isolated rat hearts were subjected to 30 min normothermic global ischemia followed by 60 min reperfusion. Hearts were harvested and analyzed for chymotrypsin-like and caspase-like activities. The values represent the mean ± SEM of 3 separate experiments and are expressed as raw data (left panels) and normalized to the 0 μmol/L ATP point (right panels).
Similar articles
- Activation of Chymotrypsin-Like Activity of the Proteasome during Ischemia Induces Myocardial Dysfunction and Death.
Sanchez G, Berrios D, Olmedo I, Pezoa J, Riquelme JA, Montecinos L, Pedrozo Z, Donoso P. Sanchez G, et al. PLoS One. 2016 Aug 16;11(8):e0161068. doi: 10.1371/journal.pone.0161068. eCollection 2016. PLoS One. 2016. PMID: 27529620 Free PMC article. - Regulation of the proteasome by ATP: implications for ischemic myocardial injury and donor heart preservation.
Majetschak M. Majetschak M. Am J Physiol Heart Circ Physiol. 2013 Aug 1;305(3):H267-78. doi: 10.1152/ajpheart.00206.2012. Epub 2013 May 24. Am J Physiol Heart Circ Physiol. 2013. PMID: 23709597 Review. - Inhibitory effect of flavonoids on 26S proteasome activity.
Chang TL. Chang TL. J Agric Food Chem. 2009 Oct 28;57(20):9706-15. doi: 10.1021/jf9017492. J Agric Food Chem. 2009. PMID: 19795881 - A subset of 26S proteasomes is activated at critically low ATP concentrations and contributes to myocardial injury during cold ischemia.
Geng Q, Romero J, Saini V, Baker TA, Picken MM, Gamelli RL, Majetschak M. Geng Q, et al. Biochem Biophys Res Commun. 2009 Dec 25;390(4):1136-41. doi: 10.1016/j.bbrc.2009.10.067. Biochem Biophys Res Commun. 2009. PMID: 19944202 Free PMC article. - Protein degradation by the 26S proteasome system in the normal and stressed myocardium.
Gomes AV, Zong C, Ping P. Gomes AV, et al. Antioxid Redox Signal. 2006 Sep-Oct;8(9-10):1677-91. doi: 10.1089/ars.2006.8.1677. Antioxid Redox Signal. 2006. PMID: 16987021 Review.
Cited by
- Impaired assembly and post-translational regulation of 26S proteasome in human end-stage heart failure.
Day SM, Divald A, Wang P, Davis F, Bartolone S, Jones R, Powell SR. Day SM, et al. Circ Heart Fail. 2013 May;6(3):544-9. doi: 10.1161/CIRCHEARTFAILURE.112.000119. Epub 2013 Mar 20. Circ Heart Fail. 2013. PMID: 23515276 Free PMC article. - Clarifying the cardiac proteasome paradox: protein quality control.
Glembotski CC. Glembotski CC. Circ Res. 2012 Aug 17;111(5):509-12. doi: 10.1161/CIRCRESAHA.112.275917. Circ Res. 2012. PMID: 22904038 Free PMC article. No abstract available. - Role of the ubiquitin-proteasome system in cardiac dysfunction of adipose triglyceride lipase-deficient mice.
Mussbacher M, Stessel H, Wölkart G, Haemmerle G, Zechner R, Mayer B, Schrammel A. Mussbacher M, et al. J Mol Cell Cardiol. 2014 Dec;77:11-9. doi: 10.1016/j.yjmcc.2014.09.028. Epub 2014 Oct 5. J Mol Cell Cardiol. 2014. PMID: 25285770 Free PMC article. - Proteostasis in cardiac health and disease.
Henning RH, Brundel BJJM. Henning RH, et al. Nat Rev Cardiol. 2017 Nov;14(11):637-653. doi: 10.1038/nrcardio.2017.89. Epub 2017 Jun 29. Nat Rev Cardiol. 2017. PMID: 28660894 Review. - Proteasome Biology: Chemistry and Bioengineering Insights.
Račková L, Csekes E. Račková L, et al. Polymers (Basel). 2020 Dec 4;12(12):2909. doi: 10.3390/polym12122909. Polymers (Basel). 2020. PMID: 33291646 Free PMC article. Review.
References
- Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. 2002;82:373–428. - PubMed
- Powell SR, Wang P, Katzeff HL, Shringarpure R, Teoh C, Khaliulin I, et al. Oxidized and ubiquitinated proteins may predict recovery of postischemic cardiac function. Essential role of the proteasome. Antioxid Redox Signal. 2005;7:538–5. - PubMed
- Liu J, Chen Q, Huang W, Horak KM, Zheng H, Mestril R, et al. Impairment of the ubiquitin-proteasome system in desminopathy mouse hearts. FASEB J. 2006;20:362–4. - PubMed
- Powell SR. The ubiquitin proteasome system in cardiac physiology and pathology. Am J Physiol Heart Circ Physiol. 2006;291:1–19. - PubMed
- Dong X, Liu J, Zheng H, Glasford JW, Huang W, Chen QH, et al. In situ dynamically monitoring the proteolytic function of the ubiquitin-proteasome system in cultured cardiac myocytes. Am J Physiol Heart Circ Physiol. 2004;287:H1417–H1425. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources