Polarised asymmetric inheritance of accumulated protein damage in higher eukaryotes - PubMed (original) (raw)
Polarised asymmetric inheritance of accumulated protein damage in higher eukaryotes
María A Rujano et al. PLoS Biol. 2006 Dec.
Abstract
Disease-associated misfolded proteins or proteins damaged due to cellular stress are generally disposed via the cellular protein quality-control system. However, under saturating conditions, misfolded proteins will aggregate. In higher eukaryotes, these aggregates can be transported to accumulate in aggresomes at the microtubule organizing center. The fate of cells that contain aggresomes is currently unknown. Here we report that cells that have formed aggresomes can undergo normal mitosis. As a result, the aggregated proteins are asymmetrically distributed to one of the daughter cells, leaving the other daughter free of accumulated protein damage. Using both epithelial crypts of the small intestine of patients with a protein folding disease and Drosophila melanogaster neural precursor cells as models, we found that the inheritance of protein aggregates during mitosis occurs with a fixed polarity indicative of a mechanism to preserve the long-lived progeny.
Conflict of interest statement
Competing interests. The authors have declared that no competing interests exist.
Figures
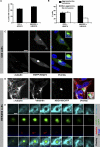
Figure 1. Polyglutamine-Expanded Proteins Form Aggresomes in Hamster O23 Cells and Human HEK293 Cells
(A) Percentage of cells containing inclusions 24 h after transfection with a fluorescently tagged huntingtin fragment containing a stretch of either 74 (O23: EGFP-HDQ74) or 119 (HEK293: HDQ119-EYFP) glutamines. (B) Fraction of cells showing either aggresome-like inclusions or non–aggresome-like inclusions (nuclear and/or multiple scattered inclusions). Bars represent standard errors of the mean. (C) Aggresome-like inclusions are either close to (upper panel) or co-localise (lower panel) with the centrosomes (decorated with γ-tubulin antibodies) in interphase O23 cells (likewise in HEK293 cells, Figure S1). (D) Vimentin microfilaments are redistributed in a cage-like manner around the inclusion, consistent with aggresome morphology. Note that also microtubules (decorated with α-tubulin antibodies) showed partial redistribution to the aggresome. (E) Sequential confocal planes of an aggresome showing both co-localisation with the centrosome and the cage of vimentin. For a full image of this cell see Figure S1B. DNA is stained with DAPI (blue) and only shown in the overlay images. Bars in (C) and (D) represent 10 μm. Bar in (E) represents 2 μm.
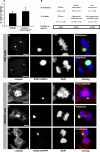
Figure 2. Aggresomes Do Not Impair Mitotic Cell Division
(A) Quantitative analysis of total mitoses in wild-type HEK293 cells and polyglutamine-expressing HEK293-HDQ119 cells. Bars represent standard error of the mean. (B) Relative fraction of mitoses in each population (diffuse, aggresome-containing, and non–aggresome-containing) of HEK293-HDQ119 cells. (C–G) Representative pictures of fixed O23 (C and D) and HEK293 (E–G) aggresome-containing cells in different mitotic phases. The aggresome is associated with only one of the poles during metaphase, anaphase, and telophase. (C) shows that alignment of chromosomes in metaphase appears normal, and (D and F) show that segregation during anaphase-telophase appears to be normal. Similarly, (C and D) show that positioning of the centrosomes is normal, and (E–F) show that distribution of microtubules and (G) cytokinesis are normal. DNA is stained with DAPI (blue). Bars, 5 μm.
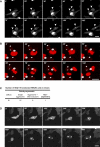
Figure 3. Aggresome Formation Does Not Impair Cell Division and Leads to Asymmetric Inheritance of Aggregated Proteins
(A) Time-lapse imaging of O23 mitotic cells expressing EGFP-HDQ74. An aggresome (indicated by the arrowhead) containing cell enters mitosis (frame 30') and completes division in 40 min (frame 70') without any apparent irregularities. The two resulting daughter cells are marked by arrowheads. See also Videos S1 and S2. (B) Time-lapse imaging of HEK293 mitotic cells expressing HDQ119-EYFP. The aggresome containing cell enters mitosis (frame 20') and like in O23 cells, completes cytokinesis (frame 90') without irregularities. Note that mitosis in HEK293 cells is slower than in O23 cells (60 ± 10 min); although the timing is normal compared to non-transfected HEK293 or cells with diffuse polyglutamine protein. See also Videos S3 and S4). (C) Distribution of mitoses in a total of 100 HDQ119 transfected HEK293 cells recorded. (D) A cell with multiple inclusions enters mitosis (frame 350') and completes cytokinesis with a delay of approximately 510 min (frame 900'). Both daughter cells inherit multiple inclusions and do not show normal morphology (see also Video S5). All images are selected frames of the time-lapse recording shown in Videos S1, S2, and S5. Videos of dividing cells without inclusions are shown in S6 (O23) and S7 (HEK293). Bar, 20 μm.
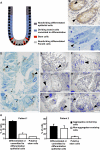
Figure 4. Polyglutamine Aggregates Are Present in Committed Crypt Cells but Absent in Stem Cells in the Small Intestine of SCA3 Patients
(A) Schematic representation of an intestinal crypt for visualisation of the different cell types present in this tissue. Light micrographs of a 4-μm (B) and 1-μm (C) section of a crypt from an SCA3 patient showing positive staining for anti-Musashi antibody. Note that stem cells are localised in between and on both sides of the morphologically recognizable Paneth cells residing at the base of the crypt. (D) A light micrograph of a crypt of a SCA3 patient showing positive staining for the anti-polyglutamine antibody IC2 in some epithelial cells (arrowheads). The asterisks (marked E–J) show representative positions of cells analyzed by subsequent electron microscopy. (E–J) show digitally modified, pseudo-coloured images of electron micrographs indicating the polyglutamine staining in blue. Differentiated epithelial cells (E), transit epithelial cells (F and I), and Paneth cells (J) contain polyglutamine aggregates (arrowheads). Stem cells (G) are negative for polyglutamine aggregates but occasionally contain micro-aggregates (H). Note that also some electron dense material is stained blue by this digital processing. In (G and H), contours are provided in black dashed lines to indicate the stem cells. Bars: D, 20 μm; E and H, 2 μm; F, 1 μm; G, I and J, 5 μm. (K) Quantification of cells with aggregates in the crypts of two SCA3 patients. As double labelling for aggregates and stem cells failed, only the stem cells that were adjacent to the Paneth cells were counted, because these could be easily identified on this basis.
Figure 5. Polyglutamine Aggregates Are Inherited by De Novo Generated Neuroblast Cells after Mitosis in D. Melanogaster
(A) Expression of Htt-Q128 (red) and Pon-GFP (green) was assessed by confocal laser scanning microscopy in whole embryos (Stage 11, in which anterior is at the top). Occasionally, Htt-Q128 aggregates were observed (inset). (B) During mitosis, the aggregated protein Htt-Q128 is associated with only one of the poles in metaphase, anaphase, and telophase, opposing the Pon-GFP crescent, indicative of asymmetric inheritance to de novo generated neuroblast. (C) Spindle pole–associated aggregates were more clearly visualised after α-tubulin (red) staining in Htt-Q128 (cyan), Pon-GFP (green) neuroblasts. DNA is stained with DAPI (blue).
Comment in
- Cellular inheritance.
Hill E. Hill E. PLoS Biol. 2006 Dec;4(12):e446. doi: 10.1371/journal.pbio.0040446. Epub 2006 Dec 5. PLoS Biol. 2006. PMID: 20076521 Free PMC article. No abstract available.
References
- Notterpek L, Ryan MC, Tobler AR, Shooter EM. PMP22 accumulation in aggresomes: Implications for CMT1A pathology. Neurobiol Dis. 1999;6:450–460. - PubMed
- Junn E, Lee SS, Suhr UT, Mouradian MM. Parkin accumulation in aggresomes due to proteasome impairment. J Biol Chem. 2002;277:47870–47877. - PubMed
- Lee HJ, Lee SJ. Characterization of cytoplasmic alpha-synuclein aggregates: Fibril formation is tightly linked to the inclusion-forming process in cells. J Biol Chem. 2002;277:48976–48983. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials