Legionella pneumophila type II secretome reveals unique exoproteins and a chitinase that promotes bacterial persistence in the lung - PubMed (original) (raw)
Legionella pneumophila type II secretome reveals unique exoproteins and a chitinase that promotes bacterial persistence in the lung
Sruti DebRoy et al. Proc Natl Acad Sci U S A. 2006.
Abstract
Type II protein secretion is critical for Legionella pneumophila infection of amoebae, macrophages, and mice. Previously, we found several enzymes to be secreted by this (Lsp) secretory pathway. To better define the L. pneumophila type II secretome, a 2D electrophoresis proteomic approach was used to compare proteins in wild-type and type II mutant supernatants. We identified 20 proteins that are type II-dependent, including aminopeptidases, an RNase, and chitinase, as well as proteins with no homology to known proteins. Because a chitinase had not been previously reported in Legionella, we determined that wild type secretes activity against both p-nitrophenyl triacetyl chitotriose and glycol chitin. An lsp mutant had a 70-75% reduction in activity, confirming the type II dependency of the secreted chitinase. Newly constructed chitinase (chiA) mutants also had approximately 75% less activity, and reintroduction of chiA restored the mutants to normal levels of activity. Although chiA mutants were not impaired for in vitro intracellular infection, they were defective upon intratracheal inoculation into the lungs of A/J mice, and antibodies against ChiA were detectable in infected animals. In contrast, mutants lacking a secreted phosphatase, protease, or one of several lipolytic enzymes were not defective in vivo. In sum, this study shows that the output of type II secretion is greater in magnitude than previously appreciated and includes previously undescribed proteins. Our data also indicate that an enzyme with chitinase activity can promote infection of a mammalian host.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
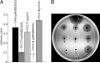
Fig. 1.
Secreted chitinase activity of L. pneumophila wild type and lspF and chiA mutants. (A) Culture supernatants of wild-type 130b (pMMB2002; black bar), lspF mutant NU275 (pMMB2002; dark gray bar), NU275 (p_lspF_; light gray bar), chiA mutant NU318 (pMMB2002; white bar), and NU318 (p_chiA_; crosshatched bar) were assayed for chitinase activity against p_-NP-[GlcNAc]3. Data represent the mean and standard deviation for triplicate cultures for each strain. The reductions in enzymatic activity for NU275 (pMMB2002) and NU318 (pMMB2002) were significant (Student's t test; P < 0.05). 130b, NU275, and NU318 behaved identically to their corresponding derivatives that contained only the cloning vector pMMB2002 (data not shown). (B) Utilization of glycol chitin by the secreted chitinase in the supernatants of strains 130b (1), 130b (pMMB2002; 2), 130b (p_chiA; 3), NU275 (4), NU275 (pMM2002; 5), NU275 (p_lspF_; 6), NU318 (7), NU318 (pMMB2002; 8), and NU318 (p_chiA_; 9). Twenty microunits of S. griseus chitinase (a) and BYE medium (b) were used as the positive and negative controls, respectively. Similar results for A and B were obtained on at least two other occasions. The independently derived chiA mutant NU319 had a phenotype identical to that of mutant NU318 (data not shown).
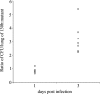
Fig. 2.
In vivo competition of L. pneumophila wild type and a chiA mutant in the lung. An equal mixture of wild-type strain 130b and chiA mutant NU318 was inoculated intratracheally into the lungs of A/J mice. The ratio of the wild type to the mutant was determined at days 1 and 3 after inoculation. Data are representative of actual values obtained per mouse (n = 5), and the solid bar indicates the mean value. The differences in the ratios of 130b and NU318 on day 1 vs. day 3 were significant (Student's t test; P < 0.05).
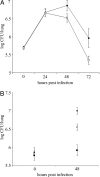
Fig. 3.
Growth and persistence of wild type and mutant L. pneumophila in the lung. A/J mice were intratracheally inoculated with equal numbers of 130b (♦; A) and the chiA mutant NU318 (□) and 130b (♦; B), NU318 (□) and the type II mutant NU275 (■), and the cfus in the infected lungs were determined at various time points. The data represent the mean and standard deviations of four to six mice for each strain. The differences between the cfus recovered for 130b and NU318 at 48 and 72 h after inoculation (A) and 130b and NU275 and 130b and NU318 at 48 h after inoculation (B) were significant (Student's t test, P < 0.01).
Similar articles
- Legionella pneumophila type II protein secretion promotes virulence in the A/J mouse model of Legionnaires' disease pneumonia.
Rossier O, Starkenburg SR, Cianciotto NP. Rossier O, et al. Infect Immun. 2004 Jan;72(1):310-21. doi: 10.1128/IAI.72.1.310-321.2004. Infect Immun. 2004. PMID: 14688110 Free PMC article. - Multiple ecto-nucleoside triphosphate diphosphohydrolases facilitate intracellular replication of Legionella pneumophila.
Riedmaier P, Sansom FM, Sofian T, Beddoe T, Schuelein R, Newton HJ, Hartland EL. Riedmaier P, et al. Biochem J. 2014 Sep 1;462(2):279-89. doi: 10.1042/BJ20130923. Biochem J. 2014. PMID: 24957128 - Structure and functional analysis of the Legionella pneumophila chitinase ChiA reveals a novel mechanism of metal-dependent mucin degradation.
Rehman S, Grigoryeva LS, Richardson KH, Corsini P, White RC, Shaw R, Portlock TJ, Dorgan B, Zanjani ZS, Fornili A, Cianciotto NP, Garnett JA. Rehman S, et al. PLoS Pathog. 2020 May 4;16(5):e1008342. doi: 10.1371/journal.ppat.1008342. eCollection 2020 May. PLoS Pathog. 2020. PMID: 32365117 Free PMC article. - Secreted phospholipases of the lung pathogen Legionella pneumophila.
Hiller M, Lang C, Michel W, Flieger A. Hiller M, et al. Int J Med Microbiol. 2018 Jan;308(1):168-175. doi: 10.1016/j.ijmm.2017.10.002. Epub 2017 Oct 28. Int J Med Microbiol. 2018. PMID: 29108710 Review. - Nutrient generation and retrieval from the host cell cytosol by intra-vacuolar Legionella pneumophila.
Price CT, Richards AM, Abu Kwaik Y. Price CT, et al. Front Cell Infect Microbiol. 2014 Aug 26;4:111. doi: 10.3389/fcimb.2014.00111. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 25207263 Free PMC article. Review. No abstract available.
Cited by
- Sel1 repeat protein LpnE is a Legionella pneumophila virulence determinant that influences vacuolar trafficking.
Newton HJ, Sansom FM, Dao J, McAlister AD, Sloan J, Cianciotto NP, Hartland EL. Newton HJ, et al. Infect Immun. 2007 Dec;75(12):5575-85. doi: 10.1128/IAI.00443-07. Epub 2007 Sep 24. Infect Immun. 2007. PMID: 17893138 Free PMC article. - Contribution of chitinases to Listeria monocytogenes pathogenesis.
Chaudhuri S, Bruno JC, Alonzo F 3rd, Xayarath B, Cianciotto NP, Freitag NE. Chaudhuri S, et al. Appl Environ Microbiol. 2010 Nov;76(21):7302-5. doi: 10.1128/AEM.01338-10. Epub 2010 Sep 3. Appl Environ Microbiol. 2010. PMID: 20817810 Free PMC article. - The Farmed Atlantic Salmon (Salmo salar) Skin-Mucus Proteome and Its Nutrient Potential for the Resident Bacterial Community.
Minniti G, Rød Sandve S, Padra JT, Heldal Hagen L, Lindén S, Pope PB, Ø Arntzen M, Vaaje-Kolstad G. Minniti G, et al. Genes (Basel). 2019 Jul 7;10(7):515. doi: 10.3390/genes10070515. Genes (Basel). 2019. PMID: 31284681 Free PMC article. - Purification of Legiobactin and importance of this siderophore in lung infection by Legionella pneumophila.
Allard KA, Dao J, Sanjeevaiah P, McCoy-Simandle K, Chatfield CH, Crumrine DS, Castignetti D, Cianciotto NP. Allard KA, et al. Infect Immun. 2009 Jul;77(7):2887-95. doi: 10.1128/IAI.00087-09. Epub 2009 Apr 27. Infect Immun. 2009. PMID: 19398549 Free PMC article.
References
- Cianciotto NP. Trends Microbiol. 2005;13:581–588. - PubMed
- Johnson TL, Abendroth J, Hol WG, Sandkvist M. FEMS Microbiol Lett. 2006;255:175–186. - PubMed
- Liles MR, Edelstein PH, Cianciotto NP. Mol Microbiol. 1999;31:959–970. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical