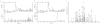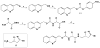Structure-activity relationship analysis of the selective inhibition of transglutaminase 2 by dihydroisoxazoles - PubMed (original) (raw)
Structure-activity relationship analysis of the selective inhibition of transglutaminase 2 by dihydroisoxazoles
R Edward Watts et al. J Med Chem. 2006.
Abstract
Human transglutaminase 2 (TG2) is believed to play an important role in the pathogenesis of various human disorders including celiac sprue, certain neurological diseases, and some types of cancer. Selective inhibition of TG2 should therefore enable further investigation of its role in physiology and disease and may lead to effective clinical treatment. Recently we showed that certain 3-halo-4-,5-dihydroisoxazole containing compounds are selective inhibitors of human TG2 with promising pharmacological activities. Here, we present definitive evidence that this class of compounds targets the active site of human TG2. Structure-activity relationship studies have provided insights into the structural prerequisites for selectivity and have led to the discovery of an inhibitor with about 50-fold higher activity than a prototypical dihydroisoxazole inhibitor with good in vivo activity. A method for preparing enantiomerically enriched analogues was also developed. Our studies show that the 5-(S)-dihydroisoxazole is a markedly better inhibitor of human TG2 than its 5-(R) stereoisomer.
Figures
Figure 1
Dihydroisoxazole inhibitors covalently bind the active site cysteine of transglutaminase 2. Human TG2 was incubated with or without inhibitor 1 before being digested with trypsin and analyzed by mass spectrometry. LC-MS chromatogram of uninhibited TG2 (A) and inhibited TG2 (B) filtered to only display peptide peaks corresponding to the predicted weight of the peptide fragment (YGQ_C_WVFAAVACTVLR) containing the active site cysteine (1787 a.m.u. (+1 peak) + 894 amu (+2 peak)) or inhibited active site cysteine (1092 amu (+2 peak)). The fragment containing the active site cysteine is the smaller peak eluting at 23.9 minutes. Note that there is a log difference in the scale of the 1092 plots. MS/MS spectrum (C) of the 1092 peak for the inhibited TG2 sample with 15 “y” and “b” peaks identified, proving the identity of the peptide fragment.
Scheme 1
General synthesis of inhibitors in this study. a) LiBH4, THF b) p-nitro-phenylchloroformate, _N_-methylmorpholine, CH2Cl2 c) 1. amino acid methyl ester, 11, DMF 2. MeOH, THF, H2O, LiOH d) EDCI, HOBT, DMF, 12.
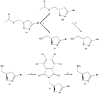
Scheme 2
Chiral resolution of dihydroisoxazoles. a) 15% v/v acetone/0.1M pH7 phosphate buffer, Amano Lipase PS b) MeOH, 20% aq. K2CO3 c) DIAD, PPh3, tetrachlorophthalimide, THF d) ethylenediamine, ACN, THF, EtOH.
Similar articles
- Transglutaminase 2 inhibitors and their therapeutic role in disease states.
Siegel M, Khosla C. Siegel M, et al. Pharmacol Ther. 2007 Aug;115(2):232-45. doi: 10.1016/j.pharmthera.2007.05.003. Epub 2007 May 13. Pharmacol Ther. 2007. PMID: 17582505 Free PMC article. Review. - Chemistry and biology of dihydroisoxazole derivatives: selective inhibitors of human transglutaminase 2.
Choi K, Siegel M, Piper JL, Yuan L, Cho E, Strnad P, Omary B, Rich KM, Khosla C. Choi K, et al. Chem Biol. 2005 Apr;12(4):469-75. doi: 10.1016/j.chembiol.2005.02.007. Chem Biol. 2005. PMID: 15850984 - Dihydroisoxazole analogs for labeling and visualization of catalytically active transglutaminase 2.
Dafik L, Khosla C. Dafik L, et al. Chem Biol. 2011 Jan 28;18(1):58-66. doi: 10.1016/j.chembiol.2010.11.004. Chem Biol. 2011. PMID: 21276939 Free PMC article. - Discovery of potent and specific dihydroisoxazole inhibitors of human transglutaminase 2.
Klöck C, Herrera Z, Albertelli M, Khosla C. Klöck C, et al. J Med Chem. 2014 Nov 13;57(21):9042-64. doi: 10.1021/jm501145a. Epub 2014 Oct 31. J Med Chem. 2014. PMID: 25333388 Free PMC article. - Recent Progress in the Development of Transglutaminase 2 (TGase2) Inhibitors.
Song M, Hwang H, Im CY, Kim SY. Song M, et al. J Med Chem. 2017 Jan 26;60(2):554-567. doi: 10.1021/acs.jmedchem.6b01036. Epub 2016 Nov 21. J Med Chem. 2017. PMID: 28122456 Review.
Cited by
- Celiac disease: prevalence, diagnosis, pathogenesis and treatment.
Gujral N, Freeman HJ, Thomson AB. Gujral N, et al. World J Gastroenterol. 2012 Nov 14;18(42):6036-59. doi: 10.3748/wjg.v18.i42.6036. World J Gastroenterol. 2012. PMID: 23155333 Free PMC article. Review. - Endoplasmic reticulum-resident protein 57 (ERp57) oxidatively inactivates human transglutaminase 2.
Yi MC, Melkonian AV, Ousey JA, Khosla C. Yi MC, et al. J Biol Chem. 2018 Feb 23;293(8):2640-2649. doi: 10.1074/jbc.RA117.001382. Epub 2018 Jan 5. J Biol Chem. 2018. PMID: 29305423 Free PMC article. - Coeliac Disease - New Pathophysiological Findings and Their Implications for Therapy.
Stein J, Schuppan D. Stein J, et al. Viszeralmedizin. 2014 Jun;30(3):156-65. doi: 10.1159/000365099. Viszeralmedizin. 2014. PMID: 26288589 Free PMC article. Review. - Transglutaminase 2 inhibitors and their therapeutic role in disease states.
Siegel M, Khosla C. Siegel M, et al. Pharmacol Ther. 2007 Aug;115(2):232-45. doi: 10.1016/j.pharmthera.2007.05.003. Epub 2007 May 13. Pharmacol Ther. 2007. PMID: 17582505 Free PMC article. Review. - Regulation of the activities of the mammalian transglutaminase family of enzymes.
Klöck C, Khosla C. Klöck C, et al. Protein Sci. 2012 Dec;21(12):1781-91. doi: 10.1002/pro.2162. Epub 2012 Nov 9. Protein Sci. 2012. PMID: 23011841 Free PMC article. Review.
References
- Fesus L, Piacentini M. Transglutaminase 2: an enigmatic enzyme with diverse functions. Trends Biochem Sci. 2002;27:534–539. - PubMed
- Lorand L, Graham RM. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol. 2003;4:140–156. - PubMed
- Kim SY, Jeitner TM, Steinert PM. Transglutaminases in disease. Neurochem Int. 2002;40:85–103. - PubMed
- Hoffner G, Djian P. Transglutaminase and Diseases of the Central Nervous System. Frontiers in Bioscience. 2005;10:3078–3092. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information