Imaging of cAMP levels and protein kinase A activity reveals that retinal waves drive oscillations in second-messenger cascades - PubMed (original) (raw)
Comparative Study
Imaging of cAMP levels and protein kinase A activity reveals that retinal waves drive oscillations in second-messenger cascades
Timothy A Dunn et al. J Neurosci. 2006.
Abstract
Recent evidence demonstrates that low-frequency oscillations of intracellular calcium on timescales of seconds to minutes drive distinct aspects of neuronal development, but the mechanisms by which these calcium transients are coupled to signaling cascades are not well understood. Here we test the hypothesis that spontaneous electrical activity activates protein kinase A (PKA). We use live-cell indicators to observe spontaneous and evoked changes in cAMP levels and PKA activity in developing retinal neurons. Expression of cAMP and PKA indicators in neonatal rat retinal explants reveals spontaneous oscillations in PKA activity that are temporally correlated with spontaneous depolarizations associated with retinal waves. In response to short applications of forskolin, dopamine, or high-potassium concentration, we image an increase in cAMP levels and PKA activity, indicating that this second-messenger pathway can be activated quickly by neural activity. Depolarization-evoked increases in PKA activity were blocked by the removal of extracellular calcium, indicating that they are mediated by a calcium-dependent mechanism. These findings demonstrate for the first time that spontaneous activity in developing circuits is correlated with activation of the cAMP/PKA pathway and that PKA activity is turned on and off on the timescale of tens of seconds. These results show a link between neural activity and an intracellular biochemical cascade associated with plasticity, axon guidance, and neural differentiation.
Figures
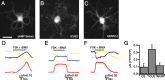
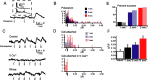
Figure 1.
Expression of functional indicators in dissociated retinal neurons. A_–_C, Fluorescence images of cultured retinal neurons expressing the cAMP sensor (A), ICUE2 (B), and AKAR2.2 (C). Retinal neurons were dissociated at P0 and imaged after 2 d in culture. Scale bar, 20 μm. D_–_F, Time course of _F_CFP (blue), _F_YFP (yellow) (imaged simultaneously), and the FRET ratio (red) of _F_YFP/_F_CFP for the CS (D), ICUE2 (E), and AKAR2.2 (F) during application of both the adenylate cyclase activator forskolin (10 μ
m
) (FSK) and phosphodiesterase inhibitor IBMX (100 μ
m
). The bar represents time of drug applications. All ratios are corrected for CFP bleed-through into YFP channel and differential bleaching of the two fluorophores. Ratio traces for CS and ICUE2 are inverted to show increases in cAMP concentration as upward deflections. G, Summary of maximal FRET ratio (Δ_R_) changes in response to both forskolin and IBMX. Δ_R_ was computed by subtracting the value of the FRET ratio averaged over five images before manipulation from the value of the FRET ratio averaged over five images around the maximum response.
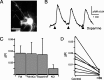
Figure 2.
AKAR2.2 responds to elevations in PKA activity induced by forskolin, dopamine, and depolarization. A, Fluorescence image of RGC in a retinal explant transfected with AKAR2.2. Scale bar, 20 μm. B, Time course of the AKAR2.2 FRET ratio change in response to short applications of dopamine (150 μ
m
) of varying duration (3 arrows correspond to 2, 4, and 8 50-ms puffs, respectively, delivered at 4 Hz). C, Summary of the AKAR2.2 FRET ratio change in response to acute application of forskolin (Fsk; 50 μ
m
, two times with 50 ms puffs at 4 Hz), forskolin in the absence of extracellular calcium (Fsk-0Ca; two times with 50 ms puffs at 4 Hz), dopamine (150 μ
m
; eight times with 50 ms puffs at 4 Hz), and high-K+ solution (105 m
m
KCl; eight times with 50 ms puffs at 4 Hz). D, The amplitude of depolarization-induced AKAR ratio changes in the absence and presence of H89, a specific PKA antagonist (25 μ
m
, after 15–25 min incubations; p < 0.0001).
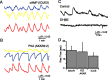
Figure 3.
Spontaneous cAMP level and PKA activity changes induced by retinal waves. A, Time course of _F_CFP (blue) and _F_YFP (yellow) recorded simultaneously and averaged over the cell body of a RGC expressing ICUE2. The ICUE2 FRET ratio is computed as _F_YFP/_F_CFP, inverted to show cAMP increases as upward deflections, corrected for CFP bleed-through into YFP channel, and corrected for differential bleaching of CFP and YFP. B, Time course of _F_CFP (blue) and _F_YFP (yellow) recorded simultaneously and averaged over the cell body of a RGC expressing AKAR2.2. The ratio is computed as _F_YFP/_F_CFP, corrected for CFP bleed-through into YFP channel, and corrected for differential bleaching. C, Blockade of retinal waves with the nicotinic acetylcholine receptor antagonist dihydro-β-erythroidine (DHβE; 10–20 μ
m
) blocked spontaneous PKA oscillations. E, Comparison of rise time of FRET ratio response to K-evoked and spontaneous events for ICUE2 and AKAR2.2.
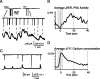
Figure 4.
Spontaneous PKA transients are temporally correlated with retinal waves. A, Simultaneous voltage clamp (top) and AKAR2.2 FRET imaging (bottom) from nearby retinal ganglion cells. Inset, Two events, a compound EPSC corresponding to a retinal wave (left) and single synaptic events (right). Wave-related events are distinguished by a slow inward current. B, The wave-triggered average indicates that a barrage of synaptic currents associated with retinal waves is followed by an increase in PKA activity and preceded by a decrease in PKA activity from the previous transient. Gray bar indicates average length of compound PSCs. C, Simultaneous voltage-clamp recording (top) and fractional change in fluorescence of the calcium indicator fura-2, loaded via its AM ester (bottom), in nearby cells. D, The wave-triggered average for Ca2+ transients indicate that barrages of synaptic currents are simultaneous with calcium increases. Gray bar indicates average length of compound PSCs.
Figure 5.
Three second depolarizations reliably evoked PKA activity transients in a calcium-dependent manner. A, Time course of AKAR2.2 FRET ratio (_F_YFP/_F_CFP) in response to 1 or 3 s applications of solutions containing 105 m
m
K+. The arrows show the starting time for an application of high-K+ solution. The numbers underlying the arrows indicate the duration of the potassium application. Inset, Current-clamp response of an RGC in response to 1 s (top) or 3 s (bottom) application of high-K+ solution. B, Histogram distribution of the change in FRET ratio (Δ_R_) in response to application of high-K+ solution of varying duration (black for 1 s duration; blue for 2 s duration; red for 3 s duration). Arrow refers to threshold over which we could reliably distinguish responses from the noise. C, Time course of AKAR2.2 FRET ratio in response to depolarization of RGC neurons induced via a cell-attached pipette in the presence and absence of 0 Ca2+ ACSF. The arrows indicate the starting time for a given depolarization pulse. The numbers below the arrows indicate the duration of the depolarization pulses. D, Histogram distribution of the change in FRET ratio (Δ_R_) to depolarization of RGC neurons induced via a cell-attached pipette of varying duration. Bottom, Histogram distribution of responses to 3 s depolarizations in the absence of extracellular calcium. Arrows refer to threshold over which we could reliably distinguish response from the noise. E, The percentage of detectable depolarization induced changes in FRET ratio computed from histogram distributions in B and D. For both potassium and electrophysiologically induced depolarizations, longer durations more reliably induced changes in FRET ratios. F, Comparison of the fractional change of fluorescence of fura-2 AM induced by increases in intracellular calcium during retinal waves and calcium increases induced by applications of high-K+ solution of varying duration. All categories are significantly different from each other (paired Student's t test, p < 0.0001).
Similar articles
- Calcium-dependent increases in protein kinase-A activity in mouse retinal ganglion cells are mediated by multiple adenylate cyclases.
Dunn TA, Storm DR, Feller MB. Dunn TA, et al. PLoS One. 2009 Nov 17;4(11):e7877. doi: 10.1371/journal.pone.0007877. PLoS One. 2009. PMID: 19924297 Free PMC article. - Multiple second-messenger system modulation of voltage-activated calcium currents in teleost retinal horizontal cells.
Pfeiffer-Linn CL, Lasater EM. Pfeiffer-Linn CL, et al. J Neurophysiol. 1998 Jul;80(1):377-88. doi: 10.1152/jn.1998.80.1.377. J Neurophysiol. 1998. PMID: 9658058 - Activation of 5-HT1A Receptors Promotes Retinal Ganglion Cell Function by Inhibiting the cAMP-PKA Pathway to Modulate Presynaptic GABA Release in Chronic Glaucoma.
Zhou X, Zhang R, Zhang S, Wu J, Sun X. Zhou X, et al. J Neurosci. 2019 Feb 20;39(8):1484-1504. doi: 10.1523/JNEUROSCI.1685-18.2018. Epub 2018 Dec 12. J Neurosci. 2019. PMID: 30541912 Free PMC article. - Imaging second messenger dynamics in developing neural circuits.
Dunn TA, Feller MB. Dunn TA, et al. Dev Neurobiol. 2008 May;68(6):835-44. doi: 10.1002/dneu.20619. Dev Neurobiol. 2008. PMID: 18383551 Free PMC article. Review. - Specificity in the cAMP/PKA signaling pathway. differential expression, regulation, and subcellular localization of subunits of PKA.
Skålhegg BS, Taskén K. Skålhegg BS, et al. Front Biosci. 1997 Jul 1;2:d331-42. doi: 10.2741/a195. Front Biosci. 1997. PMID: 9236186 Review.
Cited by
- Striatal neurones have a specific ability to respond to phasic dopamine release.
Castro LR, Brito M, Guiot E, Polito M, Korn CW, Hervé D, Girault JA, Paupardin-Tritsch D, Vincent P. Castro LR, et al. J Physiol. 2013 Jul 1;591(13):3197-214. doi: 10.1113/jphysiol.2013.252197. Epub 2013 Apr 3. J Physiol. 2013. PMID: 23551948 Free PMC article. - Spatiotemporal Dynamics of Kinase Signaling Visualized by Targeted Reporters.
Kunkel MT, Newton AC. Kunkel MT, et al. Curr Protoc Chem Biol. 2009 Dec 1;1(1):17-18. doi: 10.1002/9780470559277.ch090106. Curr Protoc Chem Biol. 2009. PMID: 21804950 Free PMC article. - The role of soluble adenylyl cyclase in neurite outgrowth.
Stiles TL, Kapiloff MS, Goldberg JL. Stiles TL, et al. Biochim Biophys Acta. 2014 Dec;1842(12 Pt B):2561-8. doi: 10.1016/j.bbadis.2014.07.012. Epub 2014 Jul 23. Biochim Biophys Acta. 2014. PMID: 25064589 Free PMC article. Review. - Fine-scale topography in sensory systems: insights from Drosophila and vertebrates.
Kaneko T, Ye B. Kaneko T, et al. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2015 Sep;201(9):911-20. doi: 10.1007/s00359-015-1022-7. Epub 2015 Jun 20. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2015. PMID: 26091779 Free PMC article. Review. - Synaptotagmin I regulates patterned spontaneous activity in the developing rat retina via calcium binding to the C2AB domains.
Chiang CW, Chen YC, Lu JC, Hsiao YT, Chang CW, Huang PC, Chang YT, Chang PY, Wang CT. Chiang CW, et al. PLoS One. 2012;7(10):e47465. doi: 10.1371/journal.pone.0047465. Epub 2012 Oct 16. PLoS One. 2012. PMID: 23091625 Free PMC article.
References
- Abdel-Majid RM, Tremblay F, Baldridge WH. Localization of adenylyl cyclase proteins in the rodent retina. Brain Res Mol Brain Res. 2002;101:62–70. - PubMed
- Adelsberger H, Garaschuk O, Konnerth A. Cortical calcium waves in resting newborn mice. Nat Neurosci. 2005;8:988–990. - PubMed
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. - PubMed
- Borodinsky LN, Root CM, Cronin JA, Sann SB, Gu X, Spitzer NC. Activity-dependent homeostatic specification of transmitter expression in embryonic neurons. Nature. 2004;429:523–530. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 EY013528/EY/NEI NIH HHS/United States
- EY016417/EY/NEI NIH HHS/United States
- R01 NS027177/NS/NINDS NIH HHS/United States
- R01 EY013528-09/EY/NEI NIH HHS/United States
- TCP00089/TI_/Telethon/Italy
- R37 NS027177/NS/NINDS NIH HHS/United States
- R21 EY016417/EY/NEI NIH HHS/United States
- DK73368/DK/NIDDK NIH HHS/United States
- F31 DK074381/DK/NIDDK NIH HHS/United States
- R01 DK073368/DK/NIDDK NIH HHS/United States
- EY13528/EY/NEI NIH HHS/United States
- NS27177/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources