A novel role for cyclooxygenase-2 in regulating vascular channel formation by human breast cancer cells - PubMed (original) (raw)
A novel role for cyclooxygenase-2 in regulating vascular channel formation by human breast cancer cells
Gargi D Basu et al. Breast Cancer Res. 2006.
Abstract
Introduction: Cyclo-oxygenase (COX)-2 expression correlates directly with highly aggressive and metastatic breast cancer, but the mechanism underlying this correlation remains obscure. We hypothesized that invasive human breast cancer cells that over-express COX-2 have the unique ability to differentiate into extracellular-matrix-rich vascular channels, also known as vasculogenic mimicry. Vascular channels have been associated with angiogenesis without involvement of endothelial cells, and may serve as another mechanism by which tumor cells obtain nutrients to survive, especially in less vascularized regions of the tumor.
Methods: To determine whether COX-2 regulates vascular channel formation, we assessed whether treatment with celecoxib (a selective COX-2 inhibitor) or silencing COX-2 synthesis by siRNA inhibits vascular channel formation by breast cancer cell lines. Cell lines were selected based on their invasive potential and COX-2 expression. Additionally, gene expression analysis was performed to identify candidate genes involved in COX-2-induced vascular channel formation. Finally, vascular channels were analyzed in surgically resected human breast cancer specimens that expressed varying levels of COX-2.
Results: We found that invasive human breast cancer cells that over-express COX-2 develop vascular channels when plated on three-dimensional matigel cultures, whereas non-invasive cell lines that express low levels of COX-2 did not develop such channels. Similarly, we identified vascular channels in high-grade invasive ductal carcinoma of the breast over-expressing COX-2, but not in low-grade breast tumors. Vascular channel formation was significantly suppressed when cells were treated with celecoxib or COX-2 siRNA. Inhibition of channel formation was abrogated by addition of exogenous prostaglandin E2. In vitro results were corroborated in vivo in tumor-bearing mice treated with celecoxib. Using gene expression profiling, we identified several genes in the angiogenic and survival pathways that are engaged in vascular channel formation.
Conclusion: Antivascular therapies targeting tumor cell vasculogenic mimicry may be an effective approach to the treatment of patients with highly metastatic breast cancer.
Figures
Figure 1
Kinetics of vascular channel formation by breast cancer cells. MDA-MB-231, MDA-MB-435, MCF-7, and ZR-75-1 cells were serum starved overnight, plated on matrigel, and images using phase contrast microscopy were taken at the times indicated. The MDA-MB-231 and MDA-MB-435 cells started to form vascular channels as early as 2 hours after plating on matrigel. Well defined patterned networks were observed by 7 hours. MCF-7 and ZR-75-1 cells failed to form patterned networks.
Figure 2
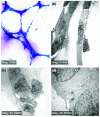
PAS staining and electron microscopic analysis of vascular channels formed by MDA-MB-231 cells. (a) PAS staining was performed on MDA-MB-231 cells plated on matrigel for 24 hours to identify the extracellular matrix secreted by cells. Pink staining refers to the glycogen and related mucopolysaccharides secreted by the cells to form the extracellular matrix-rich vascular channels. (b) Transmission electron microscopic analysis was performed on MDA-MB-231 cells plated on matrigel for 24 hours. A cluster of cells came into contact with each other to form vascular channels. There was cytoplasmic ruffling and protrusion at cell contact position. (c) There was evidence of stretching of cellular contents to form the tubular channel-like structure. (d) Absence of tight junctions between adjacent cells involved in vascular channel formation was observed. PAS, periodic acid-Schiff.
Figure 3
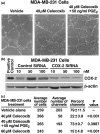
COX-2 inhibition by celecoxib or specific siRNA inhibits vascular channel formation. (a) Phase contrast images show vascular channel formation in growth factor reduced matrigel of MDA-MB-231, treated with vehicle or 40 mmol/l celecoxib. Images were captured 48 hours after plating using a phase contrast microscope. (part i) With vehicle treatment, MDA-MB-231 cells form well differentiated tubular structures. (part ii) With celecoxib treatment, differentiation into channels was significantly reduced in MDA-MB-231 cells. (part iii) Addition of 50 ng/ml PGE2 to MDA-MB-231 cells treated with 40 μM celecoxib could reverse the inhibitory effect of celecoxib. (b) COX-2 expression decreases in MDA-MB-231 cells with siRNA treatment. COX-2 protein expression was measured by Western blot. Treatment with a COX-2 siRNA for 48 hours significantly inhibited COX-2 expression at siRNA concentrations of 10, 50, and 100 nmol/l. Data shown are representative of three independent experiments. (c) Inhibition of vascular channel formation in MDA-MB-231 cells with celecoxib and COX-2-specific siRNA treatment. Quantitative analysis of vascular channel formation: the number of vascular channels was determined by counting the number of connected cells in five randomly selected fields, using 200 × magnification, and dividing that number by the total number of cells in the same field. Raw data from five standardized fields for each treatment from three separate experiments are shown. Treatment with 40 and 60 mmol/l celecoxib and treatment with a 50 nmol/l concentration of COX-2 siRNA for 48 hours caused significant decrease in the number of channels formed by MDA-MB-231 cells, and addition of 50 ng/ml of PGE2 was able to reverse the effect observed with treatment with 40 mmol/l celecoxib. P values represent significant difference between vehicle control and celecoxib treatment. COX, cyclo-oxygenase; PG, prostaglandin; siRNA, small interfering RNA.
Figure 4
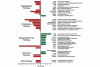
Treatment with celecoxib regulates gene expression in MDA-MB-231 cells. Differential gene expression was assessed by comparing MDA-MB-231 cells treated with or without 40 mmol/l celecoxib for 24 hours using the Affymetrix Human Genome 133A Gene Chip. Replicate microarray analyses were employed. A total of 44,760 genes were initially evaluated, and of these 1069 had a twofold or greater change. Selected results compare genes of interest associated with important cell pathways. Bars on the right represent an increase in gene expression and bars on the left indicate a decrease. COX, cyclo-oxygenase.
Figure 5
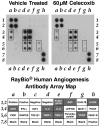
Cell supernatant analysis of angiogenic proteins revealed decreased levels with celecoxib treatment. The cell culture supernatants from MDA-MB-231 cells treated with vehicle and 40 mmol/l celecoxib for 24 hours were tested on a human angiogenesis protein array. The cell culture supernatants of cells treated with vehicle had higher amounts of GRO, IL-6, IL-8, TIMP1, TIMP2, and VEGF based on gray levels or brightness values as compared with cells treated with celecoxib. Other proteins affected by treatment were EGF, bFGF, and angiogenin. The array template is shown in the lower panel, with dark gray indicating high expression. bFGF, basic fibroblast growth factor; EGF, epidermal growth factor; IFN, interferon; IGF, insulin-like growth factor; IL, interleukin; MCP, monocyte chemoattractant protein; PDGF, platelet-derived growth factor; PG, prostaglandin; RANTES, regulated on activation, normal T cell expressed and secreted; TIMP, tissue inhibitor of matrix metalloproteinase; VEGF, vascular endothelial growth factor.
Figure 6
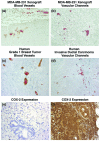
In vivo inhibition of VM with treatment and presence of VM in breast cancer specimens. (a) Vascularity of tumor xenografts in mice was evaluated by factor VIII related antigen staining (brown) for endothelial cells, and Biebrich Scarlet staining (red) for RBCs. (b) Absence of endothelial cells lining the pools of RBCs (vascular channels) was shown by no staining for factor VIII related antigen and positive staining for RBC by Biebrich Scarlet. C) Histological features of the primary human grade 1 breast tumor specimen showing blood vessels positive for CD34 staining (brown) for endothelial cells, and Biebrich Scarlet staining (red) for RBCs. No vascular channels were detected. (d) RBC pooling without the lining of endothelium (vascular channels) in the high-grade invasive ductal breast carcinoma specimen. (e, f) COX-2 staining pattern in grade 1 primary human breast tumor specimen (panel e) and in high-grade invasive ductal carcinoma specimen (penal f). Magnifications are as follows: panels a and b, 100 ×; panels c and d, 200 ×; and panels E and F, 100 × magnification). COX, cyclo-oxygenase; RBC, red blood cell; VM, vasculogenic mimicry.
Similar articles
- Mechanisms underlying the growth inhibitory effects of the cyclo-oxygenase-2 inhibitor celecoxib in human breast cancer cells.
Basu GD, Pathangey LB, Tinder TL, Gendler SJ, Mukherjee P. Basu GD, et al. Breast Cancer Res. 2005;7(4):R422-35. doi: 10.1186/bcr1019. Epub 2005 Apr 4. Breast Cancer Res. 2005. PMID: 15987447 Free PMC article. - The human cytomegalovirus-encoded chemokine receptor US28 promotes angiogenesis and tumor formation via cyclooxygenase-2.
Maussang D, Langemeijer E, Fitzsimons CP, Stigter-van Walsum M, Dijkman R, Borg MK, Slinger E, Schreiber A, Michel D, Tensen CP, van Dongen GA, Leurs R, Smit MJ. Maussang D, et al. Cancer Res. 2009 Apr 1;69(7):2861-9. doi: 10.1158/0008-5472.CAN-08-2487. Epub 2009 Mar 24. Cancer Res. 2009. PMID: 19318580 - Review: molecular pathology of cyclooxygenase-2 in cancer-induced angiogenesis.
Fosslien E. Fosslien E. Ann Clin Lab Sci. 2001 Oct;31(4):325-48. Ann Clin Lab Sci. 2001. PMID: 11688844 Review. - Cyclooxygenase as a target in lung cancer.
Brown JR, DuBois RN. Brown JR, et al. Clin Cancer Res. 2004 Jun 15;10(12 Pt 2):4266s-4269s. doi: 10.1158/1078-0432.CCR-040014. Clin Cancer Res. 2004. PMID: 15217972 Review.
Cited by
- Class I histone deacetylase inhibitor suppresses vasculogenic mimicry by enhancing the expression of tumor suppressor and anti-angiogenesis genes in aggressive human TNBC cells.
Maiti A, Qi Q, Peng X, Yan L, Takabe K, Hait NC. Maiti A, et al. Int J Oncol. 2019 Jul;55(1):116-130. doi: 10.3892/ijo.2019.4796. Epub 2019 May 6. Int J Oncol. 2019. PMID: 31059004 Free PMC article. - A pilot histomorphology and hemodynamic of vasculogenic mimicry in gallbladder carcinomas in vivo and in vitro.
Sun W, Fan YZ, Zhang WZ, Ge CY. Sun W, et al. J Exp Clin Cancer Res. 2011 Apr 29;30(1):46. doi: 10.1186/1756-9966-30-46. J Exp Clin Cancer Res. 2011. PMID: 21529356 Free PMC article. - Mechanisms of Vasculogenic Mimicry in Ovarian Cancer.
Ayala-Domínguez L, Olmedo-Nieva L, Muñoz-Bello JO, Contreras-Paredes A, Manzo-Merino J, Martínez-Ramírez I, Lizano M. Ayala-Domínguez L, et al. Front Oncol. 2019 Sep 27;9:998. doi: 10.3389/fonc.2019.00998. eCollection 2019. Front Oncol. 2019. PMID: 31612116 Free PMC article. Review. - Physicochemical aspects of the tumour microenvironment as drivers of vasculogenic mimicry.
Andreucci E, Peppicelli S, Ruzzolini J, Bianchini F, Calorini L. Andreucci E, et al. Cancer Metastasis Rev. 2022 Dec;41(4):935-951. doi: 10.1007/s10555-022-10067-x. Epub 2022 Oct 13. Cancer Metastasis Rev. 2022. PMID: 36224457 Free PMC article. Review. - Vasculogenic mimicry: a novel target for glioma therapy.
Chen YS, Chen ZP. Chen YS, et al. Chin J Cancer. 2014 Feb;33(2):74-9. doi: 10.5732/cjc.012.10292. Epub 2013 Jul 2. Chin J Cancer. 2014. PMID: 23816560 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials