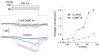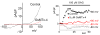Mechanosensitive ion channels and the peptide inhibitor GsMTx-4: history, properties, mechanisms and pharmacology - PubMed (original) (raw)
Review
Mechanosensitive ion channels and the peptide inhibitor GsMTx-4: history, properties, mechanisms and pharmacology
Charles L Bowman et al. Toxicon. 2007 Feb.
Abstract
Sensing the energy from mechanical inputs is ubiquitous--and perhaps the oldest form of biological energy transduction. However, the tools available to probe the mechanisms of transduction are far fewer than for the chemical and electric field sensitive transducers. The one pharmacological tool available for mechansensitive ion channels (MSCs) is a peptide (GsMTx-4) isolated from venom of the tarantula, Grammostola spatulata, that blocks cationic MSCs found in non-specialized eukaryotic tissues. In this review, we summarize the current knowledge of GsMTx-4, and discuss the inevitable crosstalk between the MSC behavior and the mechanical properties of the cell cortex.
Figures
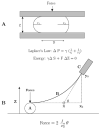
Figure 1
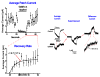
K. S. Cole’s ’flexure balance’. A. Applying force to the Arbacia egg. B. The parameters used to calculate the force (after Cole 1932a).
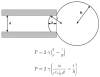
Figure 2
Aspiration of membranes into a micropipette (after (Mitchison and Swann, 1954a)). The top equation is Laplace’s law, and the bottom equation is a transformation relating the aspiration distance to the tension, γ.
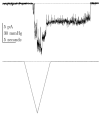
Figure 3
Endogenous mechanosensitive ion channel currents from COS 7L cells activated by a ramp of pressure. Notice the latencies in the on and off responses. (Vm = −75 mV, 100 Hz bandwidth, cell attached patch).
Figure 4
cDNA of the gene encoding GsMTx4 with the open reading frame. The first 21 amino acids are removed as a signal sequence (yellow). The protein is cleaved at an arginine (arrow) and the last two amino acids (red) are removed during amidation. The mature peptide 34 amino acid peptide is outlined in gray.
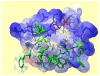
Figure 5
Solution structure of GsMTx4 determined by NMR spectroscopy. Disulfide bonds are shown in yellow, hydrophobic residues in green, acidic residues in red, and basic residues in blue. GsMTx4 has a predicted net charge of +5 at neutral pH.
Figure 6
Sequence comparison of four channel active peptides derived from spider venoms. All peptides belong to the ICK family and are all gating modifiers. Hydrophobic residues are green, charged residues red for acidic, and blue for basic. Cysteines are yellow and boxed.
Figure 7
Determination of the association and dissociation rates of GsMTx4. Left panel, washout and fitting with recovery over ~5 seconds. The data were fitted with a single exponential. Right panel, association rates. Average currents with GsMTx4 applied are subtracted from the control currents to generate a difference current. The difference current was fit with a single exponential to determine the association rate.
Figure 8
GsMTx4 is a gating modifier since the block can overcome with increasing suction. a) Average currents of an outside-out patch from rat astrocytes as a function of suction. b) The peak average currents as a function of suction showing a rightward shift for activation indicative of a gating modifier.
Figure 9
The equivalent effect of wt and enGsMTx4 on mechanosensitive ion channels. (Outside/Out patches, rat astrocytes)
Figure 10
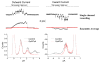
GsMTx4 affects the inward but not outward unitary currents of SACs from adult rat astrocytes. Outward currents at the left and inward currents at the right. Single channel recordings are shown underneath the pressure pulse. Ensemble averaged current in the presence (red) and absence (black) of GsMTx4. Single channel amplitude histograms show the change of unitary inward currents.
Figure 11
The effect of GsMTx4 on gramicidin gating. a) Raw channel data showing GsMTx4 increased activity. b) GsMTx4 decreased unitary conductance. c) Open channel life times increased with GsMTx4, i.e. decreased the closing rate. d) The GsMTx4 peptide decreased the closing rate of gA analogs varying in length and structure, including enantiomers.
Figure 12
GsMtx-4 doesn’t affect the AP of resting rabbit atrial cells at 8X the KD. Perforated patch (amphotericin), n=5, 37 C° (Sachs, 2004b).
Figure 13
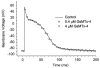
TRPC6 is a SAC and blocked by GsMTx4. CHO cells activated by the membrane permeable DAG derivative, OAG. Left panel, whole cell I/V properties showing inhibition by GsMTx4. Right panel, time course of activation by OAG (courtesy M. Spassova).
Figure 14
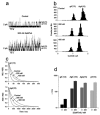
Venom gland of Grammostola spatulata. The duct to the fang is at the right. The striated muscle fibers are helically wrapped around the gland, and the angle at one position is indicated by the line. The gland is segmented into two compartments (marked segment), with contraction initiating at the left. The image is 2mm wide.
Figure 15
Hematoxylin-eosin stained section of flash frozen G. spatulata. The muscle fibers are sectioned diagonally at the right. The central duct is toward the left, but beyond the section. Beneath the muscle layer is connective tissue and then, with purple nuclei, the epithelia. The left part of the image contains the holocrine debris of the cells containing the venom. Large protein aggregates stain deep red.
Figure 16
RT PCR in situ hybridization of RNA for GsMTx4 in the columnar epithelial layer. The lumen is toward the top and the muscle toward the bottom. The granular purple stain is the RNA coding for GsMTx4, the nuclei are bluish. All cells in the epithelia seem to express GsMTx4.
Figure 17
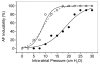
Block of dilation potentiated atrial fibrillation (AF) by 170nM GsMTx4 in the Langendorff perfused rabbit heart (Bode et al., 2001a). We have plotted how often burst stimulation resulted in AF lasting longer than 60s. Inflation increased the probability of AF (open symbols), but GsMTx4 shifted the dose-response curve to higher pressures (filled symbols).
Figure 18
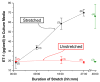
Cyclic stretch increases ET-1 secretion (Ostrow et al., 2000a).
Similar articles
- Solution structure of peptide toxins that block mechanosensitive ion channels.
Oswald RE, Suchyna TM, McFeeters R, Gottlieb P, Sachs F. Oswald RE, et al. J Biol Chem. 2002 Sep 13;277(37):34443-50. doi: 10.1074/jbc.M202715200. Epub 2002 Jun 24. J Biol Chem. 2002. PMID: 12082099 - Identification of a peptide toxin from Grammostola spatulata spider venom that blocks cation-selective stretch-activated channels.
Suchyna TM, Johnson JH, Hamer K, Leykam JF, Gage DA, Clemo HF, Baumgarten CM, Sachs F. Suchyna TM, et al. J Gen Physiol. 2000 May;115(5):583-98. doi: 10.1085/jgp.115.5.583. J Gen Physiol. 2000. PMID: 10779316 Free PMC article. - Lipid membrane interaction and antimicrobial activity of GsMTx-4, an inhibitor of mechanosensitive channel.
Jung HJ, Kim PI, Lee SK, Lee CW, Eu YJ, Lee DG, Earm YE, Kim JI. Jung HJ, et al. Biochem Biophys Res Commun. 2006 Feb 10;340(2):633-8. doi: 10.1016/j.bbrc.2005.12.046. Epub 2005 Dec 19. Biochem Biophys Res Commun. 2006. PMID: 16376854 - Spider-venom peptides that target voltage-gated sodium channels: pharmacological tools and potential therapeutic leads.
Klint JK, Senff S, Rupasinghe DB, Er SY, Herzig V, Nicholson GM, King GF. Klint JK, et al. Toxicon. 2012 Sep 15;60(4):478-91. doi: 10.1016/j.toxicon.2012.04.337. Epub 2012 Apr 20. Toxicon. 2012. PMID: 22543187 Review. - Tarantula toxins interacting with voltage sensors in potassium channels.
Swartz KJ. Swartz KJ. Toxicon. 2007 Feb;49(2):213-30. doi: 10.1016/j.toxicon.2006.09.024. Epub 2006 Sep 29. Toxicon. 2007. PMID: 17097703 Free PMC article. Review.
Cited by
- TRPV4 participates in pressure-induced inhibition of renin secretion by juxtaglomerular cells.
Seghers F, Yerna X, Zanou N, Devuyst O, Vennekens R, Nilius B, Gailly P. Seghers F, et al. J Physiol. 2016 Dec 15;594(24):7327-7340. doi: 10.1113/JP273595. Epub 2016 Dec 2. J Physiol. 2016. PMID: 27779758 Free PMC article. - Expression of stretch-activated two-pore potassium channels in human myometrium in pregnancy and labor.
Buxton IL, Singer CA, Tichenor JN. Buxton IL, et al. PLoS One. 2010 Aug 25;5(8):e12372. doi: 10.1371/journal.pone.0012372. PLoS One. 2010. PMID: 20811500 Free PMC article. - The Role of Toxins in the Pursuit for Novel Analgesics.
Maatuf Y, Geron M, Priel A. Maatuf Y, et al. Toxins (Basel). 2019 Feb 23;11(2):131. doi: 10.3390/toxins11020131. Toxins (Basel). 2019. PMID: 30813430 Free PMC article. Review. - Molecular candidates for cardiac stretch-activated ion channels.
Reed A, Kohl P, Peyronnet R. Reed A, et al. Glob Cardiol Sci Pract. 2014 Jun 18;2014(2):9-25. doi: 10.5339/gcsp.2014.19. eCollection 2014. Glob Cardiol Sci Pract. 2014. PMID: 25405172 Free PMC article. Review. - Canonical Transient Receptor Potential Channels and Their Link with Cardio/Cerebro-Vascular Diseases.
Xiao X, Liu HX, Shen K, Cao W, Li XQ. Xiao X, et al. Biomol Ther (Seoul). 2017 Sep 1;25(5):471-481. doi: 10.4062/biomolther.2016.096. Biomol Ther (Seoul). 2017. PMID: 28274093 Free PMC article. Review.
References
- Andersen OS, Nielsen C, Maer AM, Lundbæk JA, Goulian M, Koeppe RE. Ion channels as tools to monitor lipid bilayer-membrane protein interactions: gramicidin channels as molecular force transducers. Methods Enzymol. 1999;294:208–224. - PubMed
- Araque A, Carmignoto G, Haydon PG. Dynamic signaling between astrocytes and neurons. Annu Rev Physiol. 2001;63:795–813. - PubMed
- Arnon A, Hamlyn JM, Blaustein MP. Na+ entry via store-operated channels modulates Ca2+ signaling in arterial myocytes. American Journal Of Physiology-Cell Physiology. 2000;278:C163–C173. - PubMed
- Bass RB, Locher KP, Borths E, Poon Y, Strop P, Lee A, Rees DC. The structures of BtuCD and MscS and their implications for transporter and channel function. FEBS Lett. 2003;555:111–115. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources