Activation of protein kinase C modulates BACE1-mediated beta-secretase activity - PubMed (original) (raw)
Activation of protein kinase C modulates BACE1-mediated beta-secretase activity
Lizhen Wang et al. Neurobiol Aging. 2008 Mar.
Abstract
beta-Site APP cleavage enzyme 1 (BACE1) is the beta-secretase responsible for generating amyloid-beta (A beta) peptides in Alzheimer's disease (AD). Previous studies suggest that activation of protein kinase C (PKC) modulates the beta-secretase-mediated cleavage of APP and reduces the production of A beta. The mechanism of PKC-mediated modulation of beta-secretase activity, however, remains elusive. We report here that activation of PKC modulated beta-secretase activity through either suppressing the accumulation or promoting the translocation of BACE1 protein in a cell type-dependent manner. We found that activation of PKC suppressed the accumulation of BACE1 protein in fibroblasts through an enhancement of intracellular protease activities. In neurons, activation of PKC did not alter the expression level of BACE1, but led to more BACE1 translocated to the cell surface, resulting in a decreased cleavage of APP at the beta1 site. Together, Our findings provide novel mechanisms of PKC-mediated modulation of beta-secretase activity, suggesting that alteration of the intracellular trafficking of BACE1 may serve as a useful therapeutic strategy to lower the production of A beta in AD.
Figures
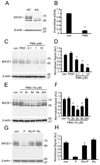
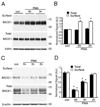
Figure 1. PMA suppressed the accumulation of BACE1 protein and the β-secretase activity in mouse fibroblasts
(A) Equal amount protein from wild type (WT) and BACE1−/− (KO) fibroblast cells were immunoblotted with antibodies against BACE1 and β-actin. (B) Treatment with PMA (10 µM) for 6 h induced a decline of BACE1-mediated β-secretase activity in fibroblasts as compared to treatment with DMSO (Con). N = 4, *p<0.0001 vs. vehicle-treated cells. Error bars represent SEM. (C) Fibroblast cells were treated with DMSO (Con), PDD (an inactive analog of PMA), or different concentration of PMA. (D) PMA suppressed the accumulation of BACE1 in a dose-dependent manner, whereas PDD or DMSO (Con) did not affect the accumulation of BACE1. N = 5 *p < 0.005 vs. vehicle-treated cells. AU stands for arbitrary unit. (E) Fibroblast cells were treated with DMSO (Con) or 10 µM PMA for different duration. (F) PMA suppressed the accumulation of BACE1 in a time-dependent manner. N = 4, *p < 0.005 vs. vehicle-treated cells. (G) Fibroblast cells were pretreated with PKC inhibitor Ro31-8220 (10 µM) (Ro) for 30 min and then treated for additional 6 h with DMSO (Con) or 10 µM PMA (P). (H) Ro31-8220 (Ro) blocked the PMA-induced suppression of BACE1 in fibroblasts. N = 4, *p< 0.0005 vs. PMA treated cells.
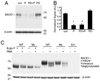
Figure 2. ERKs inhibitor did not block the reduction of BACE1 protein induced by PMA in fibroblast cells
(A) Fibroblast cells were pretreated with ERKs inhibitor PD 98095 (20 µM) (PD) for 30 min and then treated for additional 6 h with DMSO (Con) or 10 µM PMA (P). β-actin was used as loading control. (B) PD did not rescue the reduction of BACE1 protein induced by PMA in fibroblasts. N = 3, *p < 0.0005 vs. vehicle-treated cells. Error bars represent SEM. (C) Fibroblast cells transfected with empty vector (Em), wild type BACE1 (WT) and BACE1-S498A (Mu) were treated with (+) or without (−) PMA (10 µM) for 6 h. Cell lysates were also treated with (+) or without (−) _N_-glycosidase F prior to immunoblot. Noticeably, treatment with _N_-glycosidase F completely removed the sugar chains from immature and mature BACE1 protein, resulting in a predominant 50 kDa polypeptide.
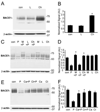
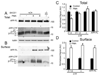
Figure 3. Intracellular protease inhibitors restored the PMA-induced degradation of BACE1 protein in fibroblast cells
(A) Fibroblast cells were treated with lactacystin (L) or chloroquine (Ch) alone for 24h prior to immunoblot with BACE1 antibody. β-actin was used as loading control. (B) Treatment with chloroquine (Ch) for 24 h blocked the degradation of BACE1 in fibroblasts. In contrast, treatment of lactacystin (L) for 24 h did not affect the accumulation of BACE1 protein in fibroblasts. N = 3, *p<0.00001 vs. vehicle-treated cells. Error bars represent SEM. (C) Fibroblast cells were pretreated with various protease inhibitors for 30 min and then co-incubated with DMSO (Con) or PMA (10 µM) (P) for 6 h. MG312 (M, 10 µM), lactacystin (L, 10 µM), and chloroquine (Ch, 50 µM) were used in this study. (D) Pretreatment with MG312 (M) or chloroquine (Ch) blocked PMA-induced suppression of BACE1 accumulation. Meanwhile, treatment with MG312 (M), chloroquine (Ch) or lactacystin (L) alone for 6 h did not affect the accumulation of BACE1 protein in fibroblast cells. N = 3, *p < 0.0005 vs. PMA-treated cells. (E) Fibroblast cells were pretreated with calpain inhibitor, calpeptin (Ca, 25 µM) or cathepsin inhibitor, cathepsin inhibitor 1 (Ct, 25uM) for 30 min and then treated for additional 6 h with 10 µM PMA. (F) Pretreatment with calpeptin (Ca) or cathepsin inhibitor 1 (Ct) blocked PMA-induced suppression of BACE1. Meanwhile, treatment with calpeptin (Ca) or cathepsin inhibitor 1 (Ct) alone did not affect the accumulation of BACE1 protein in fibroblast cells. N = 3, *p<0.0005 vs. PMA-treated cells.
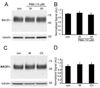
Figure 4. Treatment of PMA did not affect the accumulation of BACE1 in primary cultured neurons
(A) Treatment of neurons with vehicle (Con) or PMA (10 µM) for 3 or 6 h. Equal amount protein extracted from neurons was immunoblotted with antibodies against BACE1 and βIII-tubulin. (B) Treatment with PMA (10 µM) for 3 or 6 h did not change the accumulation of BACE1 protein in neurons as compared with the vehicle-treated control neurons (Con). N = 5. Error bars represent SEM. (C) Primary culture neurons were treated with MG-132 (M) or chloroquine (Ch) alone for 24h prior to immunoblot with a BACE1 antibody. (D) Treatment with MG-132 (M) or chloroquine (Ch) did not affect the accumulation of BACE1 in neurons. N = 3.
Figure 5. PMA induced translocation of BACE1 to the cell surface in primary cultured neurons
(A) Neurons were treated with DMSO (Con) or 10 µM PMA for 3 or 6 h and biotinylated. The biotinylated plasma membrane-bound proteins were purified and subjected to immunoblot with BACE1 antibody. β-tubulin was used as loading control. (B) Plasma membrane-bound BACE1 protein was significantly increased in neurons treated with PMA as compared with vehicle-treated neurons. N = 5, *p < 0.005 vs. vehicle-treated neurons. Error bars represent SEM. (C) Fibroblasts were treated with DMSO (Con) or 10 µM PMA for different duration and biotinylated. The biotinylated plasma membrane-bound proteins were purified and subjected to immunoblot with BACE1 antibody. (D) Both cell surface-bound and total BACE1 proteins were decreased at a similar rate in PMA-treated fibroblasts as compared with vehicle-treated cells. N = 4, *p < 0.001 vs. vehicle-treated cells.
Figure 6. Treatment of PMA reduced the accumulation of APP β1 CTF in cultured neurons
(A) Neurons were treated with DMSO (−) or 10 µM PMA for 6 h and biotinylated. The accumulation of APP full-length (APP-FL) and APP CTFs were revealed by immunoblot with an APP C-terminal antibody. β-tubulin was used as loading control. (B) The biotinylated plasma membrane-bound APP-FL and CTFs were purified and subjected to immunoblot with an APP C-terminal antibody. (C) Total APP-FL and APP CTFs were quantified and normalized by β-tubulin or APP-FL, respectively. N = 4, *p < 0.02. Error bars represent SEM. (D) Cell surface-bound APP-FL and APP CTFs were quantified and normalized against total APP-FL and APP CTFs, respectively. N = 4, *p < 0.0001.
Similar articles
- BACE1 inhibition reduces endogenous Abeta and alters APP processing in wild-type mice.
Nishitomi K, Sakaguchi G, Horikoshi Y, Gray AJ, Maeda M, Hirata-Fukae C, Becker AG, Hosono M, Sakaguchi I, Minami SS, Nakajima Y, Li HF, Takeyama C, Kihara T, Ota A, Wong PC, Aisen PS, Kato A, Kinoshita N, Matsuoka Y. Nishitomi K, et al. J Neurochem. 2006 Dec;99(6):1555-63. doi: 10.1111/j.1471-4159.2006.04178.x. Epub 2006 Nov 2. J Neurochem. 2006. PMID: 17083447 - Amyloid-β protein (Aβ) Glu11 is the major β-secretase site of β-site amyloid-β precursor protein-cleaving enzyme 1(BACE1), and shifting the cleavage site to Aβ Asp1 contributes to Alzheimer pathogenesis.
Deng Y, Wang Z, Wang R, Zhang X, Zhang S, Wu Y, Staufenbiel M, Cai F, Song W. Deng Y, et al. Eur J Neurosci. 2013 Jun;37(12):1962-9. doi: 10.1111/ejn.12235. Eur J Neurosci. 2013. PMID: 23773065 - NFkappaB-dependent control of BACE1 promoter transactivation by Abeta42.
Buggia-Prevot V, Sevalle J, Rossner S, Checler F. Buggia-Prevot V, et al. J Biol Chem. 2008 Apr 11;283(15):10037-47. doi: 10.1074/jbc.M706579200. Epub 2008 Feb 8. J Biol Chem. 2008. PMID: 18263584 - The beta-secretase, BACE: a prime drug target for Alzheimer's disease.
Vassar R. Vassar R. J Mol Neurosci. 2001 Oct;17(2):157-70. doi: 10.1385/JMN:17:2:157. J Mol Neurosci. 2001. PMID: 11816789 Review. - BACE1: the beta-secretase enzyme in Alzheimer's disease.
Vassar R. Vassar R. J Mol Neurosci. 2004;23(1-2):105-14. doi: 10.1385/JMN:23:1-2:105. J Mol Neurosci. 2004. PMID: 15126696 Review.
Cited by
- PKC ε activation prevents synaptic loss, Aβ elevation, and cognitive deficits in Alzheimer's disease transgenic mice.
Hongpaisan J, Sun MK, Alkon DL. Hongpaisan J, et al. J Neurosci. 2011 Jan 12;31(2):630-43. doi: 10.1523/JNEUROSCI.5209-10.2011. J Neurosci. 2011. PMID: 21228172 Free PMC article. - Cross-species genetic screens to identify kinase targets for APP reduction in Alzheimer's disease.
Huichalaf CH, Al-Ramahi I, Park KW, Grunke SD, Lu N, de Haro M, El-Zein K, Gallego-Flores T, Perez AM, Jung SY, Botas J, Zoghbi HY, Jankowsky JL. Huichalaf CH, et al. Hum Mol Genet. 2019 Jun 15;28(12):2014-2029. doi: 10.1093/hmg/ddz034. Hum Mol Genet. 2019. PMID: 30753434 Free PMC article. - The chaperone activity of heat shock protein 90 is critical for maintaining the stability of leucine-rich repeat kinase 2.
Wang L, Xie C, Greggio E, Parisiadou L, Shim H, Sun L, Chandran J, Lin X, Lai C, Yang WJ, Moore DJ, Dawson TM, Dawson VL, Chiosis G, Cookson MR, Cai H. Wang L, et al. J Neurosci. 2008 Mar 26;28(13):3384-91. doi: 10.1523/JNEUROSCI.0185-08.2008. J Neurosci. 2008. PMID: 18367605 Free PMC article. - Functional deprivation promotes amyloid plaque pathogenesis in Tg2576 mouse olfactory bulb and piriform cortex.
Zhang XM, Xiong K, Cai Y, Cai H, Luo XG, Feng JC, Clough RW, Patrylo PR, Struble RG, Yan XX. Zhang XM, et al. Eur J Neurosci. 2010 Feb;31(4):710-21. doi: 10.1111/j.1460-9568.2010.07103.x. Eur J Neurosci. 2010. PMID: 20384814 Free PMC article. - Beta-secretase-1 elevation in transgenic mouse models of Alzheimer's disease is associated with synaptic/axonal pathology and amyloidogenesis: implications for neuritic plaque development.
Zhang XM, Cai Y, Xiong K, Cai H, Luo XG, Feng JC, Clough RW, Struble RG, Patrylo PR, Yan XX. Zhang XM, et al. Eur J Neurosci. 2009 Dec;30(12):2271-83. doi: 10.1111/j.1460-9568.2009.07017.x. Epub 2009 Dec 10. Eur J Neurosci. 2009. PMID: 20092570 Free PMC article.
References
- Cai H, Wang Y, McCarthy D, Wen H, Borchelt DR, Price DL, Wong PC. Nat.Neurosci. 2001;4:233–234. - PubMed
- Capell A, Meyn L, Fluhrer R, Teplow DB, Walter J, Haass C. J.Biol.Chem. 2002;277:5637–5643. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases