Multiple sodium channels and their roles in electrogenesis within dorsal root ganglion neurons - PubMed (original) (raw)
Review
Multiple sodium channels and their roles in electrogenesis within dorsal root ganglion neurons
Anthony M Rush et al. J Physiol. 2007.
Abstract
Dorsal root ganglion neurons express an array of sodium channel isoforms allowing precise control of excitability. An increasing body of literature indicates that regulation of firing behaviour in these cells is linked to their patterns of expression of specific sodium channel isoforms, which have been discovered to possess distinct biophysical characteristics. The pattern of expression of sodium channels differs in different subclasses of DRG neurons and is not fixed but, on the contrary, changes in response to a variety of disease insults. Moreover, modulation of channels by their environment has been found to play an important role in the response of these neurons to stimuli. In this review we illustrate how excitability can be finely tuned to provide contrasting firing templates in different subclasses of DRG neurons by selective deployment of various sodium channel isoforms, by plasticity of expression of these proteins, and by interactions of these sodium channel isoforms with each other and with other modulatory molecules.
Figures
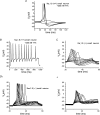
Figure 1. TTX-R currents carried by Nav1.8 have more depolarized voltage dependence than TTX-S currents in DRG neurons
Total sodium current in this DRG cell elicited using a holding potential of −100 mV and 25 ms step depolarizations to voltages ranging from −80 to +40 mV (A) can be pharmacologically separated on the basis of sensitivity to TTX (100 n
m
), allowing isolation of TTX-R currents (B) and by subtracting B from A, TTX-S currents (C). D, the voltage dependence of activation for TTX-R currents (▪) is depolarized compared to that of TTX-S currents (○) in DRG neurons. E, the voltage dependence of steady-state inactivation is more depolarized for the TTX-R currents (▪) than for TTX-S currents (○). The voltage dependence of inactivation was estimated by measuring currents elicited by 20 ms test pulses to −10 mV after 500 ms inactivating prepulses ranging from −130 to −10 mV. Adapted from data in Cummins & Waxman (1997) with permission; ©1997 by the Society for Neuroscience.
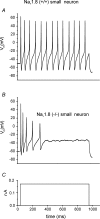
Figure 2. The TTX-R Nav1.8 channel is a major contributor to the action potential upstroke in small DRG neurons
A, TTX-R single action potentials recorded from small DRG neurons from a Nav1.8+/+ mouse, in the presence of 250 n
m
TTX. B, a longer injection of current for 1 s generates a train of TTX-R action potentials in the same neuron. C, recordings under similar conditions as in (A), but in the absence of TTX, demonstrate that only graded potentials can be elicited from ∼80% of knockout Nav1.8−/− mouse small DRG neurons. Full-sized action potentials cannot be recorded in these neurons, which lack Nav1.8 expression. Around 20% of small Nav1.8−/− DRG neurons have a more hyperpolarized RMP that allows overshooting action potentials (Da). Depolarizing the RMP in these neurons (Db, same cell as Da) inactivates the TTX-S channels and demonstrates the importance of Nav1.8 in supporting action potential firing from a depolarized RMP. Adapted from Renganathan et al. (2001) with permission; © 2001 The American Physiology Society.
Figure 3. Nav1.8 supports repetitive firing in small DRG neurons in response to sustained depolarization
Long injections of current for 1 s (C) generate a train of action potentials in Nav1.8+/+ small DRG neurons (A). The depolarized steady-state properties and rapid recovery from inactivation of the Nav1.8 channel permit this firing despite a relatively depolarized RMP. In contrast, Nav1.8−/− neurons are incapable of sustaining high frequency firing in response to identical stimuli (B). Adapted from Renganathan et al. (2001) with permission; © 2001 The American Physiology Society.
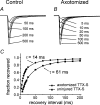
Figure 4. A rapidly repriming TTX-S sodium current, largely attributable to Nav1.3 channels, emerges in DRG neurons following transection of their peripheral axons
A, family of TTX-S sodium current recordings from intact DRG neuron, showing recovery from inactivation at −80 mV. B, family of TTX-S sodium currents from a similar neuron 7 days after peripheral axotomy, demonstrating accelerated recovery from inactivation. C, single exponential fits showing accelerated repriming following peripheral axotomy. Modified from Black et al. (1999) with permission; © 2001 The American Physiology Society.
Figure 5. Putative roles of different sodium channel subtypes in electrogenesis in small DRG neurons
Representation of subthreshold responses and overshooting action potentials are shown from small intact and axotomized DRG neurons. Under normal conditions, Nav1.7 (and Nav1.9 in some cells) is likely to bring the neuron towards threshold, and Nav1.8 is largely responsible for the overshooting action potential. Note the large depolarization necessary for action potential generation, due to the depolarized voltage dependence of activation of Nav1.8. In contrast, axotomized DRG neurons are likely to have a more hyperpolarized threshold for overshooting action potential, in line with the predominance of TTX-S (Nav1.1, Nav1.3 and Nav1.6) channels requiring less depolarization for activation and the boosted ramp currents with the presence of Nav1.3. Note that Nav1.8 and Nav1.9 are no longer involved due to down-regulation of expression after axotomy.
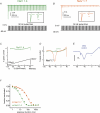
Figure 6. Distinct biophysical characteristics of the Nav1.7 sodium channel shape its role in DRG neurons
Trains of 50 Hz stimulation show that sodium channel Nav1.7 is unable to follow and sustain high frequency firing (B), when compared to, for example, Nav1.4 (A). Thus Nav1.7 is unlikely to play a major role in the action potential upstroke during repetitive firing. Using ramp stimulation to mimic small graded subthreshold potentials, Nav1.4 produces only a small response (C), in marked contrast to the large ramp current evoked with Nav1.7 (D), similar to those that can be recorded from small DRG neurons (E) (the asterisk marks a second component, blocked by cadmium and therefore likely to be due to calcium influx). F, Nav1.7 has much slower onset of inactivation than Nav1.4. Because of this, small changes in membrane potential, such as generator potentials, produce less closed-state inactivation in Nav1.7. Resistance to this form of inactivation enables Nav1.7 to respond to small, slow depolarizations close to RMP, boosting these depolarizations and bringing the cell closer to action potential threshold. Adapted from Cummins et al. (1998) with permission; ©1998 by the Society for Neuroscience.
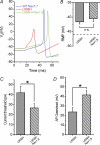
Figure 7. The erythromelalgia Nav1.7 mutation L858H inhibits firing in SCG neurons, which can be rescued by coexpression of Nav1.8 channels
When Nav1.8 is coexpressed with the Nav1.7 mutant L858H, current threshold and action potential overshoot are restored, even though the depolarization of RMP induced by L858H persists. A, suprathreshold action potentials recorded from representative superior cervical ganglion (SCG) neurons transfected with WT (blue), L858H (red) and L858H plus Nav1.8 (green) channels. B, depolarized RMP in cells with L858H channels is maintained with coexpression of Nav1.8 (P > 0.05). Dashed line indicates RMP in cells expressing WT Nav1.7 alone. C, current threshold for action potential firing is reduced by L858H coexpression with Nav1.8, when compared to L858H alone (P < 0.05). D, action potential overshoot in SCG neurons with L858H channel is increased when Nav1.8 is coexpressed with L858H (41.5 ± 4.6 mV, n = 17; P < 0.05). Thus, the introduction of Nav1.8, with depolarized activation and inactivation characteristics, allows SCG neurons to fire full overshooting action potentials, despite the depolarization induced by the L858H erythromelalgia mutation in Nav1.7. Adapted from Rush et al. (2006_a_) with permission; © 2006 National Academy of Sciences, USA.
Similar articles
- Differential action potentials and firing patterns in injured and uninjured small dorsal root ganglion neurons after nerve injury.
Zhang XF, Zhu CZ, Thimmapaya R, Choi WS, Honore P, Scott VE, Kroeger PE, Sullivan JP, Faltynek CR, Gopalakrishnan M, Shieh CC. Zhang XF, et al. Brain Res. 2004 May 29;1009(1-2):147-58. doi: 10.1016/j.brainres.2004.02.057. Brain Res. 2004. PMID: 15120592 - Contactin regulates the current density and axonal expression of tetrodotoxin-resistant but not tetrodotoxin-sensitive sodium channels in DRG neurons.
Rush AM, Craner MJ, Kageyama T, Dib-Hajj SD, Waxman SG, Ranscht B. Rush AM, et al. Eur J Neurosci. 2005 Jul;22(1):39-49. doi: 10.1111/j.1460-9568.2005.04186.x. Eur J Neurosci. 2005. PMID: 16029194 - TNF-α enhances the currents of voltage gated sodium channels in uninjured dorsal root ganglion neurons following motor nerve injury.
Chen X, Pang RP, Shen KF, Zimmermann M, Xin WJ, Li YY, Liu XG. Chen X, et al. Exp Neurol. 2011 Feb;227(2):279-86. doi: 10.1016/j.expneurol.2010.11.017. Epub 2010 Dec 9. Exp Neurol. 2011. PMID: 21145890 - Sodium channels and pain.
Waxman SG, Dib-Hajj S, Cummins TR, Black JA. Waxman SG, et al. Proc Natl Acad Sci U S A. 1999 Jul 6;96(14):7635-9. doi: 10.1073/pnas.96.14.7635. Proc Natl Acad Sci U S A. 1999. PMID: 10393872 Free PMC article. Review. - Sodium channels, excitability of primary sensory neurons, and the molecular basis of pain.
Waxman SG, Cummins TR, Dib-Hajj S, Fjell J, Black JA. Waxman SG, et al. Muscle Nerve. 1999 Sep;22(9):1177-87. doi: 10.1002/(sici)1097-4598(199909)22:9<1177::aid-mus3>3.0.co;2-p. Muscle Nerve. 1999. PMID: 10454712 Review.
Cited by
- Small-molecule natural product sophoricoside reduces peripheral neuropathic pain via directly blocking of NaV1.6 in dorsal root ganglion nociceptive neurons.
Guo W, Yang H, Wang Y, Liu T, Pan Y, Chen X, Xu Q, Zhao D, Shan Z, Cai S. Guo W, et al. Neuropsychopharmacology. 2024 Oct 16. doi: 10.1038/s41386-024-01998-w. Online ahead of print. Neuropsychopharmacology. 2024. PMID: 39414988 - Interplay of Nav1.8 and Nav1.7 channels drives neuronal hyperexcitability in neuropathic pain.
Vasylyev DV, Zhao P, Schulman BR, Waxman SG. Vasylyev DV, et al. J Gen Physiol. 2024 Nov 4;156(11):e202413596. doi: 10.1085/jgp.202413596. Epub 2024 Oct 8. J Gen Physiol. 2024. PMID: 39378238 - A Novel Antigen Design Strategy to Isolate Single-Domain Antibodies that Target Human Nav1.7 and Reduce Pain in Animal Models.
Martina M, Banderali U, Yogi A, Arbabi Ghahroudi M, Liu H, Sulea T, Durocher Y, Hussack G, van Faassen H, Chakravarty B, Liu QY, Iqbal U, Ling B, Lessard E, Sheff J, Robotham A, Callaghan D, Moreno M, Comas T, Ly D, Stanimirovic D. Martina M, et al. Adv Sci (Weinh). 2024 Oct;11(40):e2405432. doi: 10.1002/advs.202405432. Epub 2024 Aug 29. Adv Sci (Weinh). 2024. PMID: 39206821 Free PMC article. - Ion Channel and Transporter Involvement in Chemotherapy-Induced Peripheral Neurotoxicity.
Pozzi E, Terribile G, Cherchi L, Di Girolamo S, Sancini G, Alberti P. Pozzi E, et al. Int J Mol Sci. 2024 Jun 14;25(12):6552. doi: 10.3390/ijms25126552. Int J Mol Sci. 2024. PMID: 38928257 Free PMC article. Review. - Regulating neuronal excitability: The role of _S_-palmitoylation in NaV1.7 activity and voltage sensitivity.
Tang C, Duran P, Calderon-Rivera A, Loya-Lopez S, Gomez K, Perez-Miller S, Khanna R. Tang C, et al. PNAS Nexus. 2024 Jun 4;3(6):pgae222. doi: 10.1093/pnasnexus/pgae222. eCollection 2024 Jun. PNAS Nexus. 2024. PMID: 38894876 Free PMC article.
References
- Afshari FS, Ptak K, Khaliq ZM, Grieco TM, Slater NT, McCrimmon DR, Raman IM. Resurgent Na currents in four classes of neurons of the cerebellum. J Neurophysiol. 2004;92:2831–2843. - PubMed
- Akopian AN, Sivilotti L, Wood JN. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature. 1996;379:257–262. - PubMed
- Amaya F, Decosterd I, Samad TA, Plumpton C, Tate S, Mannion RJ, Costigan M, Woolf CJ. Diversity of expression of the sensory neuron-specific TTX-resistant voltage-gated sodium ion channels SNS and SNS2. Mol Cell Neurosci. 2000;15:331–342. - PubMed
- Attwell D, Cohen I, Eisner D, Ohba M, Ojeda C. The steady-state TTX-S ‘window’ sodium current in cardiac purkinje fibres. Pfugers Arch. 1979;379:137–142. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources