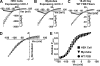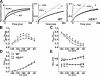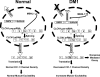Muscle chloride channel dysfunction in two mouse models of myotonic dystrophy - PubMed (original) (raw)
Muscle chloride channel dysfunction in two mouse models of myotonic dystrophy
John D Lueck et al. J Gen Physiol. 2007 Jan.
Abstract
Muscle degeneration and myotonia are clinical hallmarks of myotonic dystrophy type 1 (DM1), a multisystemic disorder caused by a CTG repeat expansion in the 3' untranslated region of the myotonic dystrophy protein kinase (DMPK) gene. Transgenic mice engineered to express mRNA with expanded (CUG)(250) repeats (HSA(LR) mice) exhibit prominent myotonia and altered splicing of muscle chloride channel gene (Clcn1) transcripts. We used whole-cell patch clamp recordings and nonstationary noise analysis to compare and biophysically characterize the magnitude, kinetics, voltage dependence, and single channel properties of the skeletal muscle chloride channel (ClC-1) in individual flexor digitorum brevis (FDB) muscle fibers isolated from 1-3-wk-old wild-type and HSA(LR) mice. The results indicate that peak ClC-1 current density at -140 mV is reduced >70% (-48.5 +/- 3.6 and -14.0 +/- 1.6 pA/pF, respectively) and the kinetics of channel deactivation increased in FDB fibers obtained from 18-20- d-old HSA(LR) mice. Nonstationary noise analysis revealed that the reduction in ClC-1 current density in HSA(LR) FDB fibers results from a large reduction in ClC-1 channel density (170 +/- 21 and 58 +/- 11 channels/pF in control and HSA(LR) fibers, respectively) and a modest decrease in maximal channel open probability(0.91 +/- 0.01 and 0.75 +/- 0.03, respectively). Qualitatively similar results were observed for ClC-1 channel activity in knockout mice for muscleblind-like 1 (Mbnl1(DeltaE3/DeltaE3)), a second murine model of DM1 that exhibits prominent myotonia and altered Clcn1 splicing (Kanadia et al., 2003). These results support a molecular mechanism for myotonia in DM1 in which a reduction in both the number of functional sarcolemmal ClC-1 and maximal channel open probability, as well as an acceleration in the kinetics of channel deactivation, results from CUG repeat-containing mRNA molecules sequestering Mbnl1 proteins required for proper CLCN1 pre-mRNA splicing and chloride channel function.
Figures
Figure 1.
Chloride channel currents from mClC-1–expressing HEK293 cells, mClC-1–expressing mouse skeletal myotubes, and native FDB fibers obtained from 18–20-d-old WT mice. Representative whole-cell currents recorded first in the absence (Raw) and then in the presence of 1 mM 9AC (9AC) using an identical voltage protocol (top). ClC-1 currents were then quantified after offline subtraction of 9AC-insensitive currents from raw currents (9AC sensitive). (Inset) Capacitative current recorded from the WT FDB fiber resulting from the average of five 10-mV depolarizations delivered from a −80-mV holding potential. The time constant the capacitative current relaxation was fitted (thick line) to a first-order exponential function (τ_m_ = 339 μs). Cell capacitance and access resistance deduced from the fit were 671 pF and 509 kΩ, respectively. The scale bars for the inset are 6 nA (vertical) and 2 ms (horizontal). The dashed lines represent the zero current level.
Figure 2.
Macroscopic properties of expressed and native ClC-1 currents. Voltage dependence of average instantaneous (open symbols) and steady-state (closed symbols) ClC-1 currents recorded from mClC-1–expressing HEK293 cells (A; n = 12), mClC-1–expressing skeletal myotubes (B; n = 4), and native FDB fibers obtained from 18–20-d-old WT mice (C; n = 8). Instantaneous currents measured in control noninjected myotubes did not display appreciable ClC-1 currents (upright triangles). (D) Superimposed and normalized instantaneous current–voltage relationships obtained from mClC-1– expressing HEK293 cells (squares), myotubes (inverted triangles), and native WT FDB fibers (circles). (E) Average relative _Po_-V curves for mClC-1–expressing HEK293 cells (squares), myotubes (inverted triangles), and native WT FDB fibers (circles) obtained from tail currents elicited at −100 mV. Smooth curves through each _PO_-V dataset were generated using a modified Boltzmann equation (Eq. 3).
Figure 3.
Deactivation kinetics of expressed and native ClC-1 channels. Voltage dependence for the relative contributions of the slow (A), fast (B), and nondeactivating (C) gating components of ClC-1 deactivation in mClC-1–expressing HEK293 cells (squares; n = 12), mClC-1–expressing myotubes (triangles; n = 4), and native FDB fibers obtained from 18–20-d-old WT mice (circles; n = 8). (D) Voltage dependence of the time constants for the fast (lower symbols) and slow (upper symbols) components of ClC-1 deactivation. *, P ≤ 0.05 HEK293 cells compared with WT FDB fibers.
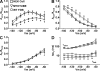
Figure 4.
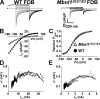
Macroscopic ClC-1 current density is dramatically reduced in FDB fibers of HSA LR mice. Representative family of ClC-1 currents recorded from FDB fibers of 19-d-old WT (A) and HSA LR (B) mice elicited using an identical voltage protocol (see Fig. 1, top). The dashed lines represent the zero current level. (C) Voltage dependence of average instantaneous current–voltage relationship in FDB fibers obtained from 18–20-d-old WT (closed circles; n = 8) and HSA LR (open squares; n = 16) mice. The apparent depolarizing shift in the reversal potential of the instantaneous ClC-1 current in FDB fibers from HSALR mice most likely arises from difficulty in resolving the true reversal potential in face of small, nearly undetectable, outward currents in these fibers. (D) Average relative _Po_-V curves for FDB fibers obtained from 18–20-d-old WT (closed circles; n = 8) and HSA LR (open squares; n = 16) mice. Smooth curves through each _PO_-V dataset were generated using a modified Boltzmann equation (Eq. 3).
Figure 5.
Deactivation kinetics of ClC-1 currents in FDB fibers obtained from WT and HSA LR mice. (A) Superimposed traces of normalized ClC-1 current deactivation (solid lines) elicited at −100 mV in FDB fibers obtained from 19-d-old WT and HSA LR mice and fit with a second order exponential (left; white dashed lines). ClC-1 currents from representative WT (middle) and HSA LR (right) FDB fibers fit with a biexponential function and replotted on log-linear plots. Fractional amplitudes of the extracted slow, fast, and nondeactivating components of the currents are shown for clarity. (B–D) Voltage dependence of the average relative contribution of the slow (B), fast (C), and steady-state (D) components of ClC-1 deactivation in FDB fibers obtained from 18–20-d-old WT (open circles, n = 8) and HSA LR (open squares, n = 16) mice. (E) Voltage dependence of the fast and slow time constants of ClC-1 deactivation in FDB fibers obtained from 18–20-d-old WT (open circles, n = 8) and HSA LR (open squares, n = 16) mice. *, P ≤ 0.05.
Figure 6.
Kinetics of ClC-1 deactivation after prepulse-dependent current reduction. (A) Representative family of ClC-1 currents (at−100 mV) recorded from an FDB fiber obtained from a 19-d-old WT mouse after 200-ms prepulses to potentials ranging from +60 to −140 mV (in 10-mV increments). (B) Voltage dependence of average time-dependent currents (Afast+Aslow) after prepulse-dependent current reduction. (Inset) Normalized ClC-1 currents (solid lines) fit with a second order exponential in control (60 mV prepulse; circles) and after ∼50% (average = 48.8 ± 0.1%, n = 8) reduction in current magnitude (−70 mV prepulse; squares). Black dashed line indicates the zero current level. (C, bottom) Voltage dependence of the average relative contribution of the fast (closed circles) and slow (open circles) components of ClC-1 deactivation (C, lower) and their respective time constants (C, top). (D) Average relative contribution of the fast and slow components of the time-dependent current (Afast+Aslow) and their respective time constants to ClC-1 deactivation (at −100 mV) in FDB fibers obtained from WT mice (+60 mV prepulse; black, n = 8), WT mice after ∼50% reduction in ClC-1 current magnitude (−70 mV prepulse; red, n = 8), HSA LR mice (+60 mV prepulse; green, n = 14), Mbnl1 ΔE3/ΔE3 mice (+60 mV prepulse; white; n = 11). *, P ≤ 0.05 compared with WT +60 mV prepulse.
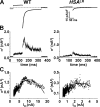
Figure 7.
Nonstationary noise analysis during ClC-1 deactivation in FDB fibers obtained from wild-type and HSA LR mice. (A) Representative mean currents in FDB fibers obtained from 12-d-old WT (left) and 18-d-old HSA LR (right) mice elicited using a 200-ms voltage step to −140 mV after a 100-ms prepulse to +60 mV. The initial 2 ms of each voltage step is blanked and the total duration of the traces truncated to 400 ms for clarity. (B) Variance time course for the currents shown in A. (C) Mean–variance relationships for the experiments shown in A and fit by Eq. 4 (see Table II for average values of fitted parameters).
Figure 8.
Macroscopic ClC-1 current density is reduced in FDB fibers from Mbnl1 ΔE3/ΔE3 mice. (A) Representative family of ClC-1 currents recorded from FDB fibers obtained from 14-d-old WT (left) and Mbn1l ΔE3/ΔE3 (right) mice. (B) Average instantaneous ClC-1 current–voltage curves for fibers obtained from 9–14-d-old WT (closed circles, n = 22) and Mbnl1 ΔE3/ΔE3 (open triangles, n = 16) mice. (C) Average relative Po-V curves for FDB fibers obtained from 9–14-d-old WT (closed circles) and Mbnl1 ΔE3/ΔE3 (open triangles) mice. Smooth curves through each _PO_-V dataset were generated using a modified Boltzmann equation (Eq. 3). (D and E) Representative mean–variance relationships for FDB fibers obtained from 12-d-old WT (D) and Mbnl1 ΔE3/ΔE3 (E) mice elicited using a 200-ms voltage step to −140 mV after a 100-ms prepulse to +60 mV (see Table II for average values of fitted parameters).
Figure 9.
Proposed molecular model for increased muscle excitability in DM1. (Left) Proper CLCN1 pre-mRNA splicing in normal skeletal muscle is regulated by muscleblind-like 1 (MBNL1) proteins, which are shown to bind to hypothetical intronic splice repressor elements in intron 6. (Right) Altered CLCN1 pre-mRNA splicing in DM1 arises from DMPK transcripts with expanded repeats (CUG)n accumulating in nuclear foci and sequestering double-stranded CUG binding proteins, including MBNL1. Depletion of MBNL1 proteins results in inclusion of additional exons (e.g., exon 7a) containing premature termination codons. Additionally, aberrantly spliced CLCN1 transcripts are subsequently exported from the nucleus, degraded through the nonsense-mediated decay pathway, and/or produce truncated proteins that potentially exert additional dominant-negative effects on ClC-1 function (Berg et al., 2004). These effects result in a dramatic reduction in the number of functional ClC-1 channels and a subsequent increase in muscle excitability.
Similar articles
- Muscleblind-Like 1 and Muscleblind-Like 3 Depletion Synergistically Enhances Myotonia by Altering Clc-1 RNA Translation.
Choi J, Personius KE, DiFranco M, Dansithong W, Yu C, Srivastava S, Dixon DM, Bhatt DB, Comai L, Vergara JL, Reddy S. Choi J, et al. EBioMedicine. 2015 Jul 31;2(9):1034-47. doi: 10.1016/j.ebiom.2015.07.028. eCollection 2015 Sep. EBioMedicine. 2015. PMID: 26501102 Free PMC article. - Expanded CUG repeats trigger aberrant splicing of ClC-1 chloride channel pre-mRNA and hyperexcitability of skeletal muscle in myotonic dystrophy.
Mankodi A, Takahashi MP, Jiang H, Beck CL, Bowers WJ, Moxley RT, Cannon SC, Thornton CA. Mankodi A, et al. Mol Cell. 2002 Jul;10(1):35-44. doi: 10.1016/s1097-2765(02)00563-4. Mol Cell. 2002. PMID: 12150905 - Age-dependent chloride channel expression in skeletal muscle fibres of normal and HSA(LR) myotonic mice.
DiFranco M, Yu C, Quiñonez M, Vergara JL. DiFranco M, et al. J Physiol. 2013 Mar 1;591(5):1347-71. doi: 10.1113/jphysiol.2012.246546. Epub 2012 Dec 17. J Physiol. 2013. PMID: 23247112 Free PMC article. - Myotonic dystrophy: emerging mechanisms for DM1 and DM2.
Cho DH, Tapscott SJ. Cho DH, et al. Biochim Biophys Acta. 2007 Feb;1772(2):195-204. doi: 10.1016/j.bbadis.2006.05.013. Epub 2006 Jun 20. Biochim Biophys Acta. 2007. PMID: 16876389 Review. - An Overview of Alternative Splicing Defects Implicated in Myotonic Dystrophy Type I.
López-Martínez A, Soblechero-Martín P, de-la-Puente-Ovejero L, Nogales-Gadea G, Arechavala-Gomeza V. López-Martínez A, et al. Genes (Basel). 2020 Sep 22;11(9):1109. doi: 10.3390/genes11091109. Genes (Basel). 2020. PMID: 32971903 Free PMC article. Review.
Cited by
- Comparison of regulated passive membrane conductance in action potential-firing fast- and slow-twitch muscle.
Pedersen TH, Macdonald WA, de Paoli FV, Gurung IS, Nielsen OB. Pedersen TH, et al. J Gen Physiol. 2009 Oct;134(4):323-37. doi: 10.1085/jgp.200910291. J Gen Physiol. 2009. PMID: 19786585 Free PMC article. - Chloride channels with ClC-1-like properties differentially regulate the excitability of dopamine receptor D1- and D2-expressing striatal medium spiny neurons.
Yarotskyy V, Lark ARS, Nass SR, Hahn YK, Marone MG, McQuiston AR, Knapp PE, Hauser KF. Yarotskyy V, et al. Am J Physiol Cell Physiol. 2022 Mar 1;322(3):C395-C409. doi: 10.1152/ajpcell.00397.2021. Epub 2022 Jan 26. Am J Physiol Cell Physiol. 2022. PMID: 35080921 Free PMC article. - From Mice to Humans: An Overview of the Potentials and Limitations of Current Transgenic Mouse Models of Major Muscular Dystrophies and Congenital Myopathies.
Sztretye M, Szabó L, Dobrosi N, Fodor J, Szentesi P, Almássy J, Magyar ZÉ, Dienes B, Csernoch L. Sztretye M, et al. Int J Mol Sci. 2020 Nov 25;21(23):8935. doi: 10.3390/ijms21238935. Int J Mol Sci. 2020. PMID: 33255644 Free PMC article. Review. - Progress in therapeutic antisense applications for neuromuscular disorders.
Aartsma-Rus A, van Ommen GJ. Aartsma-Rus A, et al. Eur J Hum Genet. 2010 Feb;18(2):146-53. doi: 10.1038/ejhg.2009.160. Epub 2009 Oct 7. Eur J Hum Genet. 2010. PMID: 19809477 Free PMC article. Review. - Defective Gating and Proteostasis of Human ClC-1 Chloride Channel: Molecular Pathophysiology of Myotonia Congenita.
Jeng CJ, Fu SJ, You CY, Peng YJ, Hsiao CT, Chen TY, Tang CY. Jeng CJ, et al. Front Neurol. 2020 Feb 11;11:76. doi: 10.3389/fneur.2020.00076. eCollection 2020. Front Neurol. 2020. PMID: 32117034 Free PMC article. Review.
References
- Bardouille, C., D. Vullhorst, and H. Jockusch. 1996. Expression of chloride channel mRNA in cultured myogenic cells: a marker of myotube maturation. FEBS Lett. 396:177–180. - PubMed
- Behrens, M.I., P. Jalil, A. Serani, F. Vergara, and O. Alverez. 1994. Possible role of apamin-sensitive K+ channels in myotonic dystrophy. Muscle Nerve. 17:1264–1270. - PubMed
- Berg, J., H. Jiang, C.A. Thornton, and S.C. Cannon. 2004. Truncated ClC-1 mRNA in myotonic dystrophy exerts a dominant-negative effect on the Cl− current. Neurology. 63:2371–2375. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R29 AR044657/AR/NIAMS NIH HHS/United States
- R01 AR046806/AR/NIAMS NIH HHS/United States
- T32DE07202/DE/NIDCR NIH HHS/United States
- AR46806/AR/NIAMS NIH HHS/United States
- K24 AR048143/AR/NIAMS NIH HHS/United States
- T32 DE007202/DE/NIDCR NIH HHS/United States
- AR44657/AR/NIAMS NIH HHS/United States
- R01 AR044657/AR/NIAMS NIH HHS/United States
- AR050762/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases