Generation and characterization of human monoclonal neutralizing antibodies with distinct binding and sequence features against SARS coronavirus using XenoMouse - PubMed (original) (raw)
Generation and characterization of human monoclonal neutralizing antibodies with distinct binding and sequence features against SARS coronavirus using XenoMouse
Melissa Coughlin et al. Virology. 2007.
Abstract
Passive therapy with neutralizing human monoclonal antibodies (mAbs) could be an effective therapy against severe acute respiratory syndrome coronavirus (SARS-CoV). Utilizing the human immunoglobulin transgenic mouse, XenoMouse, we produced fully human SARS-CoV spike (S) protein specific antibodies. Antibodies were examined for reactivity against a recombinant S1 protein, to which 200 antibodies reacted. Twenty-seven antibodies neutralized 200TCID(50) SARS-CoV (Urbani). Additionally, 57 neutralizing antibodies were found that are likely specific to S2. Mapping of the binding region was achieved with several S1 recombinant proteins. Most S1 reactive neutralizing mAbs bound to the RBD, aa 318-510. However, two S1 specific mAbs reacted with a domain upstream of the RBD between aa 12 and 261. Immunoglobulin gene sequence analyses suggested at least 8 different binding specificities. Unique human mAbs could be used as a cocktail that would simultaneously target several neutralizing epitopes and prevent emergence of escape mutants.
Figures
Fig. 1
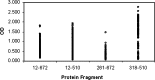
Reactivity of HmAbs produced from hybridomas generated from immunized XenoMouse® against S1-Ig fragments by ELISA. All S-V5-HIS reactive Abs were tested against S1-Ig (12–672) coated recombinant protein. The 200 Abs that reacted with S1-Ig (12–672) were then tested for reactivity against three S1-Ig fragments (12–510, 261–510 and 318–510).
Fig. 2
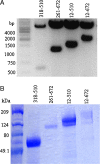
Expression of overlapping fragments of the S1 domain of SARS-CoV S protein. (A) Four plasmid constructs encoding different fragments of the S1 protein (12–672, 12–510, 261–672, 318–510) were transformed into MC 1061/P3 cells and insert size confirmed by digestion with _Nhe_I and _Bam_H1 and analyzed on a 1% agarose gel. (B) Recombinant protein expression in transiently transfected 293T cells was confirmed by Coomassie Blue staining of a 4–20% SDS/PAGE gel.
Fig. 3
Neutralizing mAbs were purified and examined for S1-Ig fragment reactivity in an ELISA. Recombinant proteins were coated on ELISA plates and increasing dilutions of mAb tested for reactivity to the relevant S1 recombinant protein. (A) Group 1A purified mAbs reactivity to 318–510 recombinant protein. (B) Group 1B purified mAbs reactivity to 318–510 recombinant protein. (C) Group 1D purified mAb reactivity to 318–510 recombinant protein. (D) Goup 2B purified mAbs reactivity to 12–510 recombinant protein.
Fig. 4
Alignment of CDR sequences of neutralizing mAbs. Immunoglobulin genes of neutralizing antibodies were sequenced. Alignment of the amino acid sequences of the heavy chain variable region (A) and light chain variable region (B) of all mAbs are depicted and arranged by common gene segment usage. Additions in mAb sequences not contained in germ line sequence are annotated (#) in germ line sequence. N/A specific gene segment could not be identified.
Similar articles
- Molecular and biological characterization of human monoclonal antibodies binding to the spike and nucleocapsid proteins of severe acute respiratory syndrome coronavirus.
van den Brink EN, Ter Meulen J, Cox F, Jongeneelen MA, Thijsse A, Throsby M, Marissen WE, Rood PM, Bakker AB, Gelderblom HR, Martina BE, Osterhaus AD, Preiser W, Doerr HW, de Kruif J, Goudsmit J. van den Brink EN, et al. J Virol. 2005 Feb;79(3):1635-44. doi: 10.1128/JVI.79.3.1635-1644.2005. J Virol. 2005. PMID: 15650189 Free PMC article. - Human monoclonal antibody combination against SARS coronavirus: synergy and coverage of escape mutants.
ter Meulen J, van den Brink EN, Poon LL, Marissen WE, Leung CS, Cox F, Cheung CY, Bakker AQ, Bogaards JA, van Deventer E, Preiser W, Doerr HW, Chow VT, de Kruif J, Peiris JS, Goudsmit J. ter Meulen J, et al. PLoS Med. 2006 Jul;3(7):e237. doi: 10.1371/journal.pmed.0030237. PLoS Med. 2006. PMID: 16796401 Free PMC article. - Neutralizing human monoclonal antibodies to severe acute respiratory syndrome coronavirus: target, mechanism of action, and therapeutic potential.
Coughlin MM, Prabhakar BS. Coughlin MM, et al. Rev Med Virol. 2012 Jan;22(1):2-17. doi: 10.1002/rmv.706. Epub 2011 Sep 8. Rev Med Virol. 2012. PMID: 21905149 Free PMC article. Review. - SARS vaccine development.
Jiang S, He Y, Liu S. Jiang S, et al. Emerg Infect Dis. 2005 Jul;11(7):1016-20. doi: 10.3201/1107.050219. Emerg Infect Dis. 2005. PMID: 16022774 Free PMC article. Review.
Cited by
- Analysis of B Cell Receptor Repertoires Reveals Key Signatures of the Systemic B Cell Response after SARS-CoV-2 Infection.
Zhang Y, Yan Q, Luo K, He P, Hou R, Zhao X, Wang Q, Yi H, Liang H, Deng Y, Hu F, Li F, Liu X, Feng Y, Li P, Qu L, Chen Z, Pan-Hammarström Q, Feng L, Niu X, Chen L. Zhang Y, et al. J Virol. 2022 Feb 23;96(4):e0160021. doi: 10.1128/JVI.01600-21. Epub 2021 Dec 8. J Virol. 2022. PMID: 34878902 Free PMC article. - Perspectives on therapeutic neutralizing antibodies against the Novel Coronavirus SARS-CoV-2.
Zhou G, Zhao Q. Zhou G, et al. Int J Biol Sci. 2020 Mar 15;16(10):1718-1723. doi: 10.7150/ijbs.45123. eCollection 2020. Int J Biol Sci. 2020. PMID: 32226289 Free PMC article. Review. - Recent advances in therapeutic applications of neutralizing antibodies for virus infections: an overview.
Ali MG, Zhang Z, Gao Q, Pan M, Rowan EG, Zhang J. Ali MG, et al. Immunol Res. 2020 Dec;68(6):325-339. doi: 10.1007/s12026-020-09159-z. Epub 2020 Nov 8. Immunol Res. 2020. PMID: 33161557 Free PMC article. Review. - Neutralizing monoclonal antibodies against highly pathogenic coronaviruses.
Xiang R, Wang Y, Wang L, Deng X, Huo S, Jiang S, Yu F. Xiang R, et al. Curr Opin Virol. 2022 Apr;53:101199. doi: 10.1016/j.coviro.2021.12.015. Epub 2021 Dec 30. Curr Opin Virol. 2022. PMID: 35038651 Free PMC article. Review. - Rapid discovery and optimization of therapeutic antibodies against emerging infectious diseases.
Rogers J, Schoepp RJ, Schröder O, Clements TL, Holland TF, Li JQ, Li J, Lewis LM, Dirmeier RP, Frey GJ, Tan X, Wong K, Woodnutt G, Keller M, Reed DS, Kimmel BE, Tozer EC. Rogers J, et al. Protein Eng Des Sel. 2008 Aug;21(8):495-505. doi: 10.1093/protein/gzn027. Epub 2008 May 13. Protein Eng Des Sel. 2008. PMID: 18480090 Free PMC article.
References
- Baker S.C. Coronaviruses from common colds to severe acute respiratory syndrome. Pediatr. Infect. Dis. J. 2004;23:1049–1050. - PubMed
- Berry J.D., Jones S., Drebot M.A., Andonov M., Sabara X.Y., Yuan H., Weingartl L., Fernando P., Marszal J., Gren B., Nicolas M., Andonova F., Ranada M.J., Gubbins T.B., Ball P., Kitcdhing Y., Li A., Kabani F., Plummer F. Development and characterization of neutralizing monoclonal antibody to the SARS-coronavirus. J. Virol. Methods. 2004;120:87–96. - PMC - PubMed
- Chan P.K.S., Tang J.W., Hui D.S.C, SARS: clinical presentation, transmission, pathogenesis and treatment options. Clin. Sci. 2006;110:193–204. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous