Release of outer membrane vesicles by Gram-negative bacteria is a novel envelope stress response - PubMed (original) (raw)
Release of outer membrane vesicles by Gram-negative bacteria is a novel envelope stress response
Amanda J McBroom et al. Mol Microbiol. 2007 Jan.
Abstract
Conditions that impair protein folding in the Gram-negative bacterial envelope cause stress. The destabilizing effects of stress in this compartment are recognized and countered by a number of signal transduction mechanisms. Data presented here reveal another facet of the complex bacterial stress response, release of outer membrane vesicles. Native vesicles are composed of outer membrane and periplasmic material, and they are released from the bacterial surface without loss of membrane integrity. Here we demonstrate that the quantity of vesicle release correlates directly with the level of protein accumulation in the cell envelope. Accumulation of material occurs under stress, and is exacerbated upon impairment of the normal housekeeping and stress-responsive mechanisms of the cell. Mutations that cause increased vesiculation enhance bacterial survival upon challenge with stressing agents or accumulation of toxic misfolded proteins. Preferential packaging of a misfolded protein mimic into vesicles for removal indicates that the vesiculation process can act to selectively eliminate unwanted material. Our results demonstrate that production of bacterial outer membrane vesicles is a fully independent, general envelope stress response. In addition to identifying a novel mechanism for alleviating stress, this work provides physiological relevance for vesicle production as a protective mechanism.
Figures
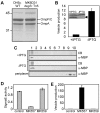
Fig. 1. Vesicle production is not directly correlated with activity of the σE pathway
A. Diagrams indicating locations of transposon insertion sites (Kan), membrane anchor (MA), protease, PDZ protein interaction, and signal sequence (SS) domains for the MK6D31 and MK11F26 overvesiculation mutants. The insertion site in MK6D31 would cause an eight-amino-acid truncation of DegS, while the MK11F26 mutant insertion within the signal sequence of degP effectively creates a full null. B. β-Galactosidase assays measuring transcription of lacZ from the σE-responsive rpoHP3::lacZ promoter in ADA600 transduced with mutations affecting vesiculation. ADA600 with pWSK130 (low-copy KanR) is the control. Mutants are listed from the highest vesiculation phenotype (MK8A44 nlpI::Tn5) to the lowest (MK9G12 glnA::Tn5) based on vesicle OMP quantification (McBroom et al., 2006). Cultures were assayed after growth at 37°C to an OD600 of 0.3. *P < 0.05, **P < 0.01.
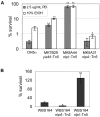
Fig. 2. Reduced DegS function impairs σE activation and increases vesicle production
A. Porin profiles of purified outer membranes prepared from OD-matched cultures of wild-type DH5α and MK6D31 degS::Tn5. Samples from equal culture densities were assayed by SDS-PAGE and Coomassie Blue staining. B. Vesicle production of DegS-depletable strain CAG43248 grown with (DegS expressed) or without (DegS depleted) IPTG. Values are relative to the culture grown with IPTG. (Inset) DegS depletion in the absence of IPTG shown by immunoblot of equivalent amounts of cell lysates at vesicle harvest. DegS is indicated (arrowhead); the upper band is a non-specific loading control. C. Equilibrium density gradients of vesicles from DegS-expressed and DegS-depleted cultures. Periplasm from DH5α was applied to the same gradient as a control for non-vesicle-associated MBP. Fraction 1 is the least dense; fraction 10 is the most dense. Prominent vesicle proteins were visualized by Coomassie Blue (CB) stained SDS-PAGE; MBP was visualized by immunoblotting (α-MBP). D and E. σE activity levels at the time of vesicle harvest (D) and vesicle production (E) of wild-type ADA600 and degS::Tn5 transductants MK557 and MK559. Values are relative to the ADA600 control.
Fig. 3. Vesicle production is modulated in response to envelope stress
A. Vesicle production of DH5α and MK11F26 degP::Tn5 with no plasmid, vector (pCS19), or DegP plasmid (pCS20) grown at 37°C. Where indicated, cultures contained 10 μM IPTG; values are relative to DH5α. B. Vesicle production of DH5α and MK11F26 degP::Tn5 at 30°C, 34°C and 37°C. Each set is normalized to the DH5α control for that temperature. (Inset) Vesicle production of DH5α at 30°C, 34°C and 37°C; values are relative to the 30°C condition. C. Vesicle production of DH5α with vector (pCS19) or DegP plasmid (pCS20) at 37°C. Where indicated, cultures contained 10 μM IPTG; values are relative to DH5α with vector. D. σE activity at the time of vesicle harvest in cultures of ADA600 corresponding to conditions in C. Values are relative to ADA600 with vector. E. Vesicle production of MK11F26 degP::Tn5 with vector (pCS19) or DegP plasmid (pCS20) at 30°C. Where indicated, cultures contained 10 μM IPTG; values are relative to DH5α with vector. F. Vesicle production of DH5α with vector (pHDB67) or MBP expression plasmid (pJH68). Cultures contained 0.05% or 0.2% arabinose; values are relative to DH5α with vector at the corresponding induction level. For all panels *P < 0.05, **P < 0.01 are indicated for instances where differences are not obviously significant. G–I. Samples of vesicles from cultures described in A, C and F (0.2% arabinose induction), respectively, analysed by SDS-PAGE and Coomassie Blue staining. Bands corresponding to OMPs F/C and A, DegP and MBP are labelled; loading volume dilution factors in G are indicated.
Fig. 4. Increased vesicle production enhances bacterial survival under stress
A. Survival of wild-type DH5α, a mutant with moderately increased vesiculation (MK7B29 yieM::Tn5), a mutant with highly increased vesiculation (MK8A44 nlpI::Tn5), and a mutant with reduced vesiculation (MK5A31 nlpA::Tn5) after 2 h of exposure to 10% ethanol (white bars) or 2.5 μg ml−1 polymyxin B (grey bars). B. Survival of WBS164 carrying the maltose-inducible lamB-lacZX90 allele in comparison with transductants of WBS164 with either the nlpA::Tn5 mutation causing vesicle underproduction or the nlpI::Tn5 mutation causing vesicle overproduction. Expression of the toxic LamB-LacZX90 fusion protein was induced for 2 h with 0.02% maltose. For both panels *P < 0.05, **P < 0.005.
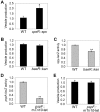
Fig. 5. Vesiculation is fully independent of all other known envelope stress responses
A. Vesicle production of TR51 (cpxR::spc) relative to its isogenic parent MC4100 (WT). B and C. Vesicle production (B) and spy-lacZ promoter activity at the time of vesicle harvest (C) of TR886 (baeR::kan) relative to its isogenic parent TR530 (WT). spy promoter activity is only partially regulated by the Bae pathway, but the reduction in activity is apparent. D and E. Psp pathway activity as measured by pspA-lacZ promoter activity at the time of vesicle harvest (D) and vesicle production (E) of MK789 (pspF::mTn_10-tet_) relative to its isogenic parent MVA4 (WT). For all panels *P < 0.05, **P < 0.005.
Fig. 6. Vesiculation allows preferential packaging and elimination of cargo from the cell envelope
A. Representative total OD-matched culture samples of wild-type rpoHP3::lacZ reporter strain CAG16037 expressing WT cyt or cyt-YYF assayed by SDS-PAGE and immunoblotting for cytochrome (upper panel) and MBP (lower panel). Relative densitometry values for cytochrome and MBP content are indicated. The quantity of each species present in the WT cyt-expressing culture sample is set to 1.0. B. Representative vesicles from cultures of CAG16037 expressing WT cyt and cyt-YYF analysed by SDS-PAGE and Coomassie Blue staining (upper panel) or immunoblotting for MBP (lower panel). Relative densitometry values and cyt/MBP ratios are indicated. Experimental average for fold cyt-YYF enrichment is 10.2 ± 1.5 SEM.
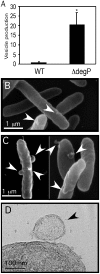
Fig. 7. Stress-induced vesiculation in Gram-negative bacteria
A. Vesicle production of WT Salmonella ATCC 14028 and ATCC 14028 degP::cat grown at 37°C; values are relative to WT. *P < 0.05. B and C. Scanning electron micrographs of DH5α (B) and MK11F26 degP::Tn5 (C) grown at 37°C. D. Thin-section transmission electron microscopy of degP::Tn5 grown at 30°C overexpressing DegP from pCS20 induced with 10 μM IPTG. Vesicles are indicated (arrows).
Similar articles
- Outer membrane vesicle production by Escherichia coli is independent of membrane instability.
McBroom AJ, Johnson AP, Vemulapalli S, Kuehn MJ. McBroom AJ, et al. J Bacteriol. 2006 Aug;188(15):5385-92. doi: 10.1128/JB.00498-06. J Bacteriol. 2006. PMID: 16855227 Free PMC article. - Modulation of bacterial outer membrane vesicle production by envelope structure and content.
Schwechheimer C, Kulp A, Kuehn MJ. Schwechheimer C, et al. BMC Microbiol. 2014 Dec 21;14:324. doi: 10.1186/s12866-014-0324-1. BMC Microbiol. 2014. PMID: 25528573 Free PMC article. - Improved methods for producing outer membrane vesicles in Gram-negative bacteria.
Henry T, Pommier S, Journet L, Bernadac A, Gorvel JP, Lloubès R. Henry T, et al. Res Microbiol. 2004 Jul-Aug;155(6):437-46. doi: 10.1016/j.resmic.2004.04.007. Res Microbiol. 2004. PMID: 15249060 - Bacterial outer membrane vesicles and the host-pathogen interaction.
Kuehn MJ, Kesty NC. Kuehn MJ, et al. Genes Dev. 2005 Nov 15;19(22):2645-55. doi: 10.1101/gad.1299905. Genes Dev. 2005. PMID: 16291643 Review. - Role of the Gram-Negative Envelope Stress Response in the Presence of Antimicrobial Agents.
Guest RL, Raivio TL. Guest RL, et al. Trends Microbiol. 2016 May;24(5):377-390. doi: 10.1016/j.tim.2016.03.001. Epub 2016 Apr 5. Trends Microbiol. 2016. PMID: 27068053 Review.
Cited by
- Enhanced glutathione content allows the in vivo synthesis of fluorescent CdTe nanoparticles by Escherichia coli.
Monrás JP, Díaz V, Bravo D, Montes RA, Chasteen TG, Osorio-Román IO, Vásquez CC, Pérez-Donoso JM. Monrás JP, et al. PLoS One. 2012;7(11):e48657. doi: 10.1371/journal.pone.0048657. Epub 2012 Nov 21. PLoS One. 2012. PMID: 23185270 Free PMC article. - Quantitative and qualitative preparations of bacterial outer membrane vesicles.
Chutkan H, Macdonald I, Manning A, Kuehn MJ. Chutkan H, et al. Methods Mol Biol. 2013;966:259-72. doi: 10.1007/978-1-62703-245-2_16. Methods Mol Biol. 2013. PMID: 23299740 Free PMC article. - Envelope control of outer membrane vesicle production in Gram-negative bacteria.
Schwechheimer C, Sullivan CJ, Kuehn MJ. Schwechheimer C, et al. Biochemistry. 2013 May 7;52(18):3031-40. doi: 10.1021/bi400164t. Epub 2013 Apr 25. Biochemistry. 2013. PMID: 23521754 Free PMC article. - Mechanisms of Antimicrobial Peptide Resistance in Gram-Negative Bacteria.
Band VI, Weiss DS. Band VI, et al. Antibiotics (Basel). 2015 Mar;4(1):18-41. doi: 10.3390/antibiotics4010018. Antibiotics (Basel). 2015. PMID: 25927010 Free PMC article. - Bacterial membrane vesicles: formation, functions, and roles in bacterial-phage interactions.
Xuan S, Xuan G. Xuan S, et al. World J Microbiol Biotechnol. 2024 Sep 21;40(10):329. doi: 10.1007/s11274-024-04148-y. World J Microbiol Biotechnol. 2024. PMID: 39304539 Review.
References
- Ades SE. Control of the alternative sigma factor sigmaE in Escherichia coli. Curr Opin Microbiol. 2004;7:157–162. - PubMed
- Alba BM, Gross CA. Regulation of the Escherichia coli sigma-dependent envelope stress response. Mol Microbiol. 2004;52:613–619. - PubMed
- Alba BM, Zhong HJ, Pelayo JC, Gross CA. degS (hhoB) is an essential Escherichia coli gene whose indispensable function is to provide sigmaE activity. Mol Microbiol. 2001;40:1323–1333. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases