Duty, desire or indifference? A qualitative study of patient decisions about recruitment to an epilepsy treatment trial - PubMed (original) (raw)
Duty, desire or indifference? A qualitative study of patient decisions about recruitment to an epilepsy treatment trial
Krysia Canvin et al. Trials. 2006.
Abstract
Background: Epilepsy is a common neurological condition, in which drugs are the mainstay of treatment and drugs trials are commonplace. Understanding why patients might or might not opt to participate in epilepsy drug trials is therefore of some importance, particularly at a time of rapid drug development and testing; and the findings may also have wider applicability. This study examined the role of patient perceptions in the decision-making process about recruitment to an RCT (the SANAD Trial) that compared different antiepileptic drug treatments for the management of new-onset seizures and epilepsy.
Methods: In-depth interviews with 23 patients recruited from four study centres. All interviews were tape-recorded and transcribed; the transcripts were analysed thematically using a qualitative data analysis package.
Results: Of the nineteen informants who agreed to participate in SANAD, none agreed for purely altruistic reasons. The four informants who declined all did so for very specific reasons of self-interest. Informants' perceptions of the nature of the trial, of the drugs subject to trial, and of their own involvement were all highly influential in their decision-making. Informants either perceived the trial as potentially beneficial or unlikely to be harmful, and so agreed to participate; or as potentially harmful or unlikely to be beneficial and so declined to participate.
Conclusion: Most patients applied 'weak altruism', while maintaining self-interest. An emphasis on the safety and equivalence of treatments allowed some patients to be indifferent to the question of involvement. There was evidence that some participants were subject to 'therapeutic misconceptions'. The findings highlight the individual nature of trials but nonetheless raise some generic issues in relation to their design and conduct.
Figures
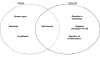
Figure 1
Reasons for agreeing or declining to participate SANAD.
Similar articles
- Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.
Crider K, Williams J, Qi YP, Gutman J, Yeung L, Mai C, Finkelstain J, Mehta S, Pons-Duran C, Menéndez C, Moraleda C, Rogers L, Daniels K, Green P. Crider K, et al. Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article. - Exploring the motivations of patients with type 2 diabetes to participate in clinical trials: a qualitative analysis.
Estcourt S, Epton J, Epton T, Vaidya B, Daly M. Estcourt S, et al. Res Involv Engagem. 2016 Dec 12;2:34. doi: 10.1186/s40900-016-0050-y. eCollection 2016. Res Involv Engagem. 2016. PMID: 29507768 Free PMC article. - Processes in recruitment to randomised controlled trials of medicines for children (RECRUIT): a qualitative study.
Shilling V, Williamson PR, Hickey H, Sowden E, Smyth RL, Young B. Shilling V, et al. Health Technol Assess. 2011 Mar;15(15):1-116. doi: 10.3310/hta15150. Health Technol Assess. 2011. PMID: 21443838 - Developing decision support tools incorporating personalised predictions of likely visual benefit versus harm for cataract surgery: research programme.
Sparrow JM, Grzeda M, Frost A, Liu C, Johnston RL, Scanlon P, Pithara C, Elliott D, Donovan J, Joseph-Williams N, Holland-Hart D, Donachie PHJ, Dixon P, Kandiyali R, Taylor H, Breheny K, Sterne J, Hollingworth W, Evans D, Fox F, Theodoropoulou S, Hughes R, Quinn M, Gray D, Benjamin L, Loose A, Edwards L, Craggs P, Paget F, Kapoor K, Searle J. Sparrow JM, et al. Southampton (UK): National Institute for Health and Care Research; 2022 Oct. Southampton (UK): National Institute for Health and Care Research; 2022 Oct. PMID: 36322691 Free Books & Documents. Review. - Antiepileptic drug monotherapy for epilepsy: a network meta-analysis of individual participant data.
Nevitt SJ, Sudell M, Weston J, Tudur Smith C, Marson AG. Nevitt SJ, et al. Cochrane Database Syst Rev. 2017 Jun 29;6(6):CD011412. doi: 10.1002/14651858.CD011412.pub2. Cochrane Database Syst Rev. 2017. PMID: 28661008 Free PMC article. Updated. Review.
Cited by
- Challenges of maintaining research protocol fidelity in a clinical care setting: a qualitative study of the experiences and views of patients and staff participating in a randomized controlled trial.
Lawton J, Jenkins N, Darbyshire JL, Holman RR, Farmer AJ, Hallowell N. Lawton J, et al. Trials. 2011 May 4;12:108. doi: 10.1186/1745-6215-12-108. Trials. 2011. PMID: 21542916 Free PMC article. Clinical Trial. - Reasons for participating in randomised controlled trials: conditional altruism and considerations for self.
McCann SK, Campbell MK, Entwistle VA. McCann SK, et al. Trials. 2010 Mar 22;11:31. doi: 10.1186/1745-6215-11-31. Trials. 2010. PMID: 20307273 Free PMC article. - Does a video clip enhance recruitment into a parenting trial? Learnings from a study within a trial.
Mattock HC, Ryan R, O'Farrelly C, Babalis D, Ramchandani PG. Mattock HC, et al. Trials. 2020 Oct 15;21(1):856. doi: 10.1186/s13063-020-04779-0. Trials. 2020. PMID: 33059763 Free PMC article. - Motivation and frustration in cardiology trial participation: the patient perspective.
Meneguin S, Cesar LA. Meneguin S, et al. Clinics (Sao Paulo). 2012;67(6):603-8. doi: 10.6061/clinics/2012(06)10. Clinics (Sao Paulo). 2012. PMID: 22760899 Free PMC article.
References
- Prescott R, Counsell C, Gillespie W, Grant AM, Russell IT, Kiauka S, Colthart IR, Ross S, Shepherd SM, Russell D. Factors that limit the quality, number and progress of randomised controlled trials. Health Technol Assess. 1999;3 - PubMed
LinkOut - more resources
Full Text Sources